Metal and Microbial Contamination in the Water Available for Agriculture in Selected Farm Settlements in Nigeria
| Received 30 Mar, 2023 |
Accepted 26 Aug, 2023 |
Published 30 Sep, 2023 |
Background and Objective: Several diseases have been reportedly linked to the consumption of water with metal and/or microbial contamination, or agricultural produce watered by such method. This study assessed the water available for agriculture in three selected farm settlements in Southwestern Nigeria. Materials and Methods: As 15, 12 and 5 water samples from the Ajegunle, Akufo and Eruwa farm settlements, respectively were analyzed for 11 heavy and trace metals and 7 microbial populations, using standard laboratory procedures, for dry and wet seasons. Analysis of Variance (ANOVA) and Duncan’s Multiple Range Tests were done using SPSS, version 20. Pollution sources were apportioned using Principal Component Analysis while seasonal spatial variation maps were generated via the Inverse Density Weighted method. Results: Water from the three farm settlements showed possibilities of cadmium, iron, manganese, E. coli and streptococcal contaminations. The pollution sources identified were bedrock weathering, fertilizer/agricultural waste/run-off leachates, agricultural activities, fecal contamination, livestock agricultural wastes, sewage effluents and organic decomposition. Spatial maps showing the existing distributions of selected metals and microbial populations within Ajegunle and Akufo farm settlements were produced. Conclusion: This research has generated information for the eradication or substantial reduction of the potential harm of metal and microbial contamination to the farm settlers and consumers of their agricultural produce.
| Copyright © 2023 Folarin et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Over time, there have been reports of pollution from high levels of heavy and trace metals in different media as well as negative health effects on humans resulting from consumption via the food chain1. The use of contaminated water for irrigation and livestock farming has been considered to be responsible for several disease outbreaks after consumption of such produce2,3. Hence the need to assess and monitor their concentrations in plant tissues and animal meat to curb their excessive build-up in the human food chain1,4,5.
Researchers have used multivariate statistical analyses such as principal component analysis (PCA) and cluster analysis (CA) to analyze water quality and apportion pollution sources6-9. Also, geographic information system (GIS), using different techniques, such as inverse distance weighted (IDW) interpolation, etc. has been used for site suitability analyses and inventory data management, solute transport and leaching studies, assessment of groundwater contamination vulnerability and modeling of groundwater flow as well as spatial mapping by integrating water quality data with spatial data10,11. Spatial groundwater variation maps have been generated by Sahoo et al.12 and Gidey13 among others.
This study assessed the contamination, apportioned pollution sources using principal component analysis (PCA) and mapped the spatial variabilities of heavy and trace metals as well as microbial populations in the water samples collected from the Ajegunle, Akufo and Eruwa farm settlements.
Despite many years of cultivation and use of fertilizers in these predominantly agricultural farm settlements, information on heavy and trace metal concentrations as well as microbial populations and their potential dangers were not available till the time this research was conducted. The findings from this research will enhance good and systematic utilization of the water within the settlements in such a way as to eradicate, or at least minimize the potentially harmful effects of metal and microbial contamination in plants, animals and eventual human consumers.
MATERIALS AND METHODS
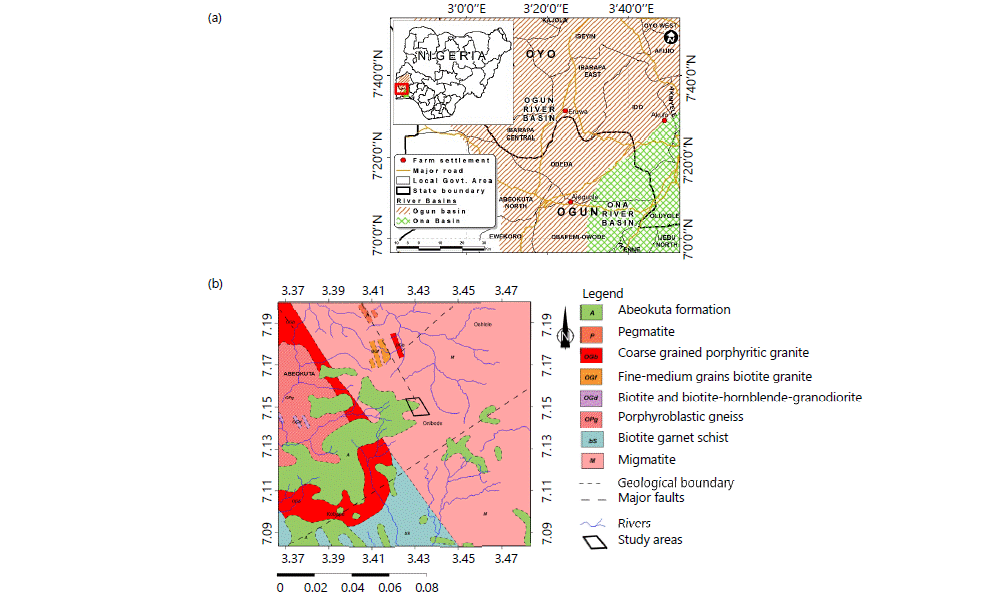
Study area: This study was conducted in three selected farm settlements within the Southwestern part of Nigeria, Ajegunle, Akufo and Eruwa farm settlements (Fig. 1a). Akufo and Eruwa are in Ido and Ibarapa East Local Government of Oyo State, respectively while Ajegunle farm settlement is in the Obafemi Owode Local Government of Ogun State.
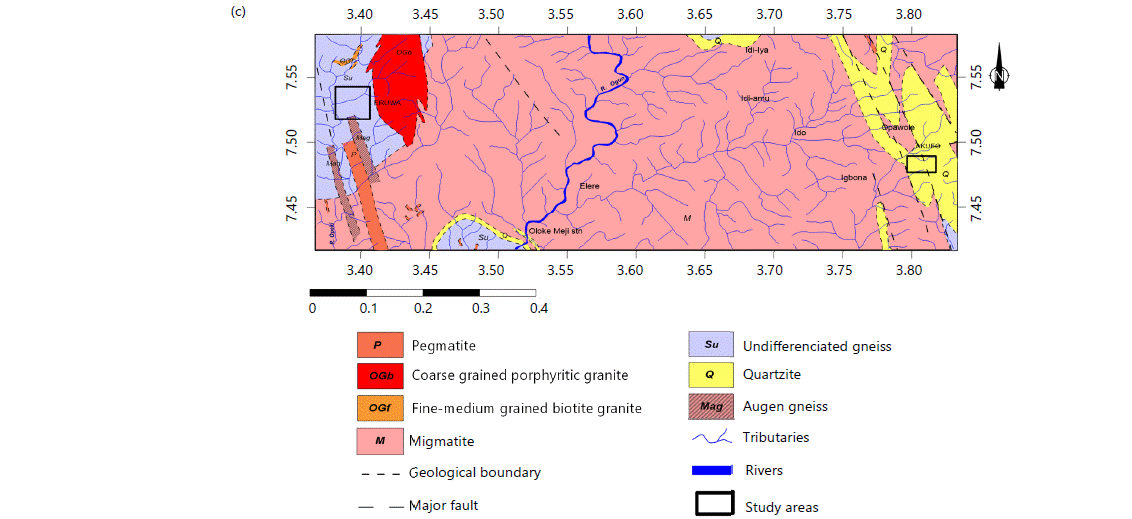
The three farm settlements are located within the Ogun River Basin, within Latitudes 6°26'N and 9°10'N and Longitudes 2°28'E and 4°8'E. The basin is of generally low relief with a North-South direction gradient and experiences two seasons: Dry from November to March and wet from April to October14,15. The three farm settlements fall within the basement complex terrain (Fig. 1b and c). The Abeokuta formation and migmatite underlie the Ajegunle farm settlement while Akufo and Eruwa are underlain with quartzite and undifferentiated gneiss, respectively9,16.
The Ajegunle farm settlers are majorly livestock farmers in poultry, fish farming and piggery, but they also grow maize and cassava. The Akufo farm settlers grow cassava, yam, oil palm, kola nut, cocoa and timber as well as do poultry farming and cattle rearing. In the Eruwa farm settlement, the prevalent crops are cassava, vegetables, cashew and fruits such as watermelon while they also engage in livestock farming such as piggery. These farm settlements have a long history of over 60 years of cultivation of staple crops and use of pesticides and fertilizers as well as other veterinary drugs and agrochemicals.
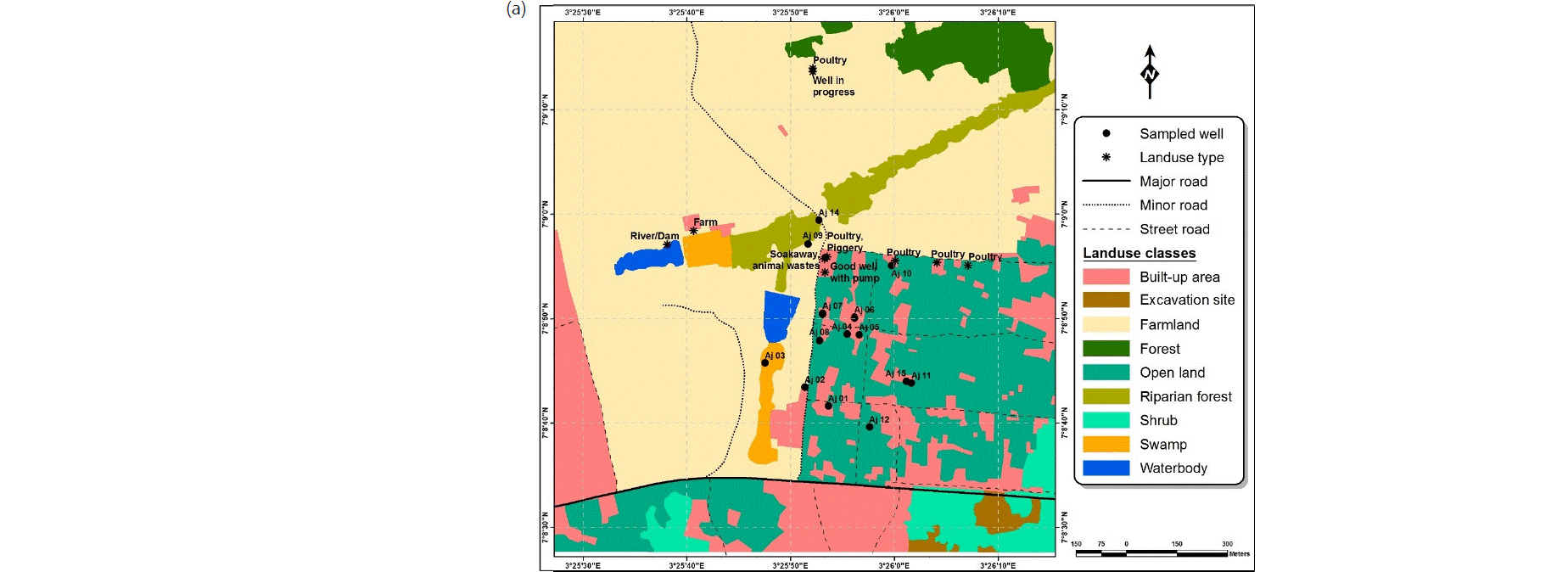
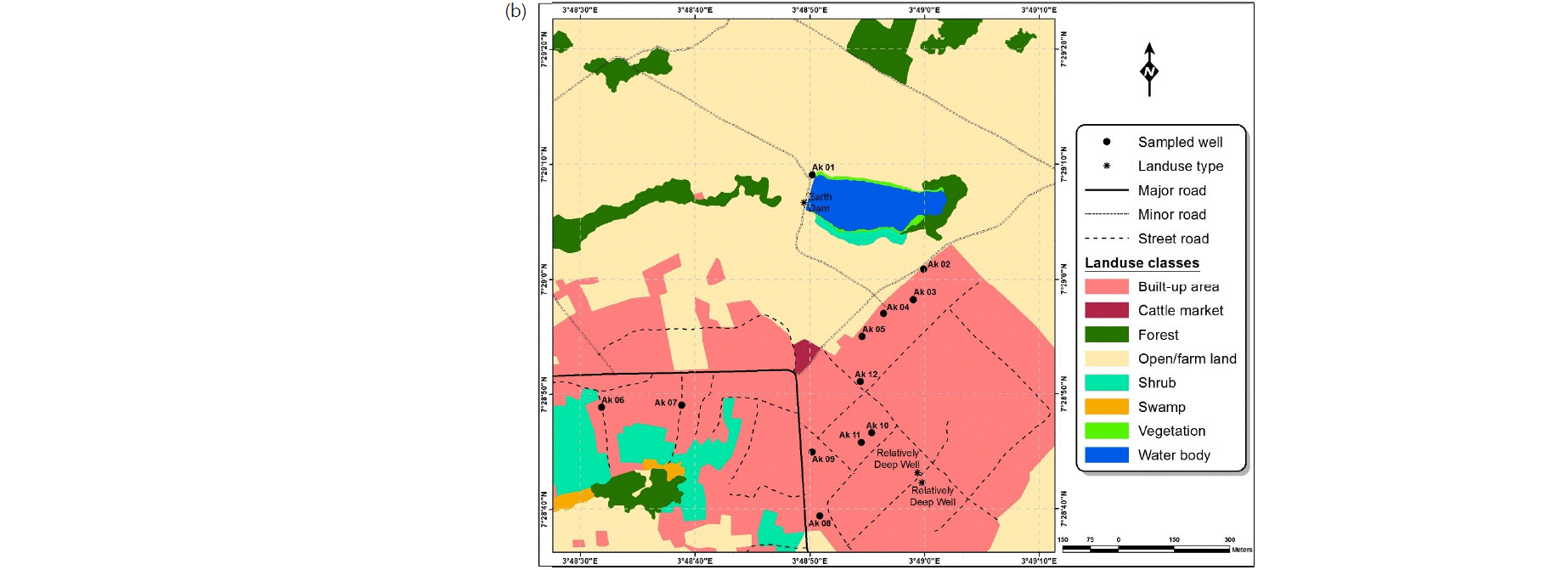
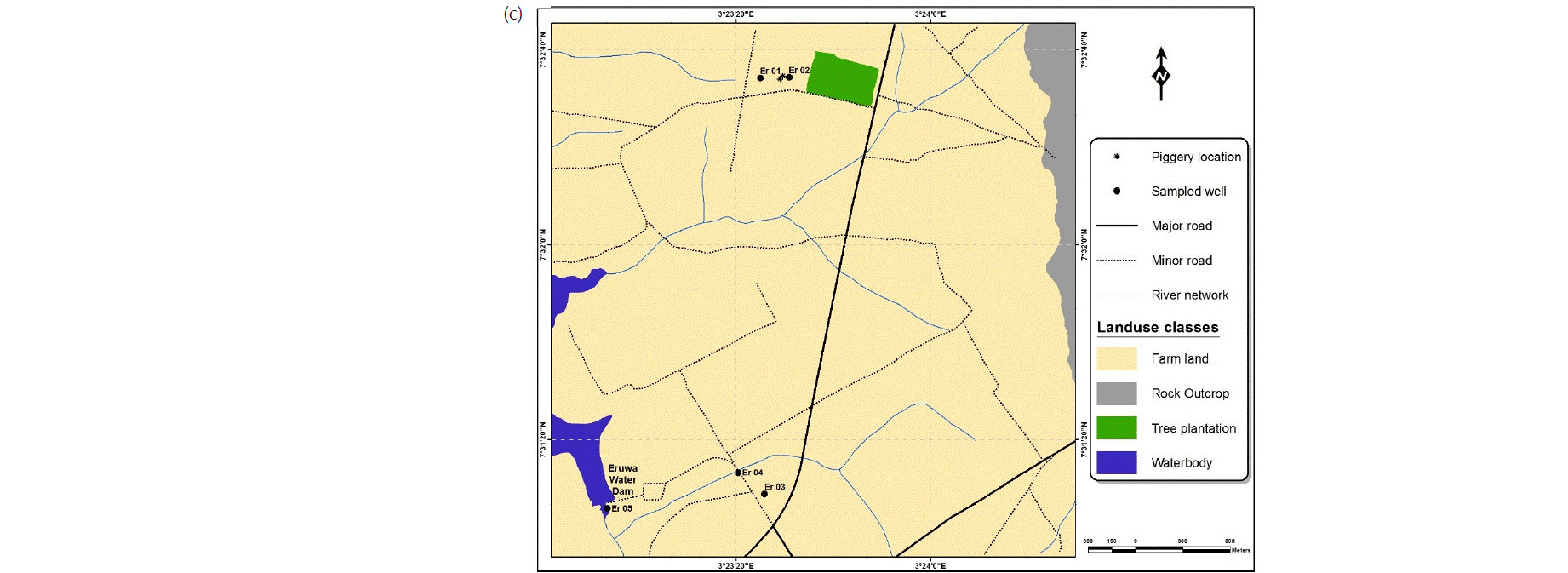
Water sampling: Water samples were collected in conformity with standard procedures17 from the Ajegunle, Akufo and Eruwa farm settlements, 15, 12 and 5 from hand-dug wells and the main earth dams/rivers/dams, respectively (Fig. 2a-c). The samples were collected for analysis during both the wet and dry seasons between August, 2016 and April, 2017. The latitudes, longitudes and elevations of the sampling points were measured with an eTrex 10 Garmin handheld GPS device.
|
|
Laboratory analyses: The water samples were analyzed for Lead, Cadmium, Zinc, Manganese, Copper, Chromium, Aluminium, Arsenic, Mercury, Boron and Iron, as well as for Coliform count, Total Bacteria Count, E. Coli count, Salmonella count, Vibrio cholera, S. aureus and Streptococcal feacal, using standard laboratory procedures. The concentrations of the metals were determined using an Atomic Absorption Spectrophotometer (AAS), model BUCK 200 while the Multiple Fermentation Tube/Most Probable Number (MPN) method was used for the microbial analyses17-21. The measurements were done in duplicates and the averages computed and recorded.
Statistical analysis: The heavy and trace metal concentrations as well as the microbial population counts from the laboratory analyses of the water samples were subjected to descriptive statistics and the results compared with the World Health Organization22 and the Nigerian Standard for Drinking Water Quality23 standards. Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test were done using SPSS, version 20. The results are presented as means±standard error (S.E.) at a 5% level of significance.
PCA pollution source apportionment: Heavy and trace metal pollution, as well as microbial contamination sources, were apportioned via principal component analysis (PCA), using SPSS, version 20. The PCA was carried out after the necessary standardization and application of the Kaiser-Mayer-Olkin (KMO) measure of sampling adequacy. Only components with eigenvalues greater than 1.00 were retained as the extracted components24-28.
IDW spatial variation mapping: This was achieved by geo-referencing and digitizing the base maps via the Arc GIS software, version 10 (2015). The inverse distance weighted (IDW) interpolation technique was used to generate and integrate spatial and attribute databases. Spatial variation maps of the heavy and trace metals, as well as the microbial populations, were then generated for the wet and dry seasons to show the patterns and seasonal variabilities within Ajegunle and Akufo farm settlements29,30. The sampling points within the Eruwa farm settlement were very few and very wide apart, hence spatial variation maps were not generated for the settlement using IDW.
RESULTS
Means and standard deviations: The means and standard deviations, minimum and maximum values for heavy and trace metal concentrations (mg L–1) and microbial populations (×105 CFU mL–1) in the water samples collected within Ajegunle farm settlement are presented in Table 1.
The means and standard deviations, minimum and maximum values for heavy and trace metal concentrations (mg L–1) and microbial populations (×105 CFU mL–1) in the water samples collected within the Akufo farm settlement are presented in Table 2.
The means and standard deviations, minimum and maximum values for heavy and trace metal concentrations (mg L–1) and microbial populations (x105 CFU mL–1) in the water samples collected in the Eruwa farm settlement are presented in Table 3
Principal component analysis
Factor loadings: The result of the principal component analysis of heavy and trace metals in the water samples collected from the Ajegunle farm settlement revealed 4 extracted components while that of microbial populations showed only 2 extracted components (Table 4).
The result of the principal component analysis of heavy and trace metals in the water samples collected from the Akufo farm settlement showed 2 extracted components while that of microbial populations showed only 1 extracted component (Table 5).
The result of the principal component analysis of heavy and trace metals in the water samples collected from the Eruwa farm settlement revealed only 1 extracted component while that of microbial populations showed 2 extracted components (Table 6).
| Table 1: | Metal concentrations and microbial populations in the water samples from Ajegunle Farm Settlement | |||
| Heavy and trace metal (mg L–1) |
Dry season | Wet season | WHO |
NSDWQ |
Dry season ------------------ |
Wet season ------------------ |
||
(2011) |
(2007) |
Min |
Max |
Min |
Max |
|||
| Lead | 0.005±0.005a | 0.004±0.005a | 0.010±0.000 |
0.010±0.000 |
0.01 |
0.01 |
0.01 |
0.01 |
| Cadmium | 0.002± 0.004a | 0.004±0.005a | 0.003±0.000 |
0.003±0.000 |
0.001 |
0.01 |
0.001 |
0.01 |
| Zinc | 0.530±0.106a | 0.352±0.077b | - |
3.000±0.000 |
0.34 |
0.66 |
0.13 |
1.13 |
| Manganese | 0.017±0.005a | 0.048±0.028a | 0.400±0.000 |
0.200±0.000 |
0.01 |
0.02 |
0.01 |
0.1 |
| Copper | 0.010±0.004a | 0.050±0.054a | 2.000±0.000 |
1.000±0.000 |
0.01 |
0.02 |
0.01 |
0.14 |
| Chromium | Not detected | Negligible | 0.0500±0.0000 |
0.0500±0.0000 |
- |
- |
- |
- |
| Aluminium | Not detected | Negligible | 0.1000±0.0000 |
0.2000±0.0000 |
- |
- |
- |
- |
| Arsenic | Not detected | Negligible | 0.0100±0.0000 |
0.0100±0.0000 |
- |
- |
- |
- |
| Mercury | Not detected | Negligible | 0.0060±0.0000 |
0.0010±0.0000 |
- |
- |
- |
- |
| Boron | Not detected | Negligible | 2.4000±0.0000 |
- |
- |
- |
- |
- |
| Iron | 0.268±0.121a | 0.617±0.290a | 0.100±0.000 |
0.200±0.000 |
0.27 |
0.36 |
0.13 |
1.15 |
| Coliform count | 0.185±0.069a | 0.143±0.096a | - |
10.000±0.000 |
0.1 |
0.3 |
0.1 |
0.3 |
| Total bacteria count | 1.069±0.253a | 0.896±0.304a | - |
- |
0.6 |
1.4 |
0.4 |
1.3 |
| E. coli count | 0.177±0.060a | 0.129±0.080b | - |
0.000±0.000 |
0.1 |
0.3 |
0.1 |
0.3 |
| S. aureus count | 0.308±0.104a | 0.293±0.149b | - |
- |
0.2 |
0.5 |
0.1 |
0.6 |
| Streptococcal count | 0.262±0.051a | 0.061±0.056a | - |
0.000±0.000 |
0.2 |
0.3 |
0.1 |
0.15 |
| Values represent mean±standard deviation (SD), values along the same row with different superscripts are significantly different at p<0.05 level, the means and standard deviations, minimum and maximum values for heavy and trace metal concentrations (mg L–1) and microbial populations (×105 CFU mL–1) in the water samples collected within the Akufo farm settlement were presented in Table 2 | ||||||||
| Table 2: | Metal concentrations and microbial populations in the water samples from Akufo farm settlement | |||
| Heavy and trace metal (mg L–1) |
Dry season | Wet season | WHO |
NSDWQ |
Dry season ------------------ |
Wet season ------------------ |
||
(2011) |
(2007) |
Min |
Max |
Min |
Max |
|||
| Lead | 0.0050 ±0.0054a | 0.0093±0.0099a | 0.0100±0.0000 |
0.0100±0.0000 |
0.01 |
0.01 |
0.001 |
0.03 |
| Cadmium | 0.0015±0.0035a | 0.0075±0.0062a | 0.0030±0.0000 |
0.0030±0.0000 |
0.001 |
0.01 |
0.01 |
0.02 |
| Zinc | 0.3563±0.0867a | 1.2808±0.1569b | - |
3.0000±0.0000 |
0.25 |
0.51 |
1.12 |
1.61 |
| Manganese | 0.0350±0.0120a | 1.3558±0.2413b | 0.4000±0.0000 |
0.2000±0.0000 |
0.02 |
0.05 |
0.75 |
1.6 |
| Copper | 0.0075±0.0046a | 0.4133±0.1028b | 2.0000±0.0000 |
1.0000±0.0000 |
0.01 |
0.01 |
0.23 |
0.62 |
| Chromium | 0.0003±0.0005a | 0.0002±0.0004a | 0.0500±0.0000 |
0.0500±0.0000 |
- |
- |
- |
- |
| Aluminium | 0.0001±0.0003a | 0.0000±0.0000a | 0.1000±0.0000 |
0.2000±0.0000 |
- |
- |
- |
- |
| Arsenic | 0.0001±0.0003a | 0.0000±0.0000a | 0.0100±0.0000 |
0.0100±0.0000 |
- |
- |
- |
- |
| Mercury | 0.0002±0.0004a | 0.0000±0.0000a | 0.0060±0.0000 |
0.0010±0.0000 |
- |
- |
- |
- |
| Boron | 0.0002±0.0004a | 0.0000±0.0000a | 2.4000±0.0000 |
- |
- |
- |
- |
- |
| Iron | 0.2988±0.0412a | 1.5675±0.1764b | 0.1000±0.0000 |
0.2000±0.0000 |
0.24 |
0.36 |
1.27 |
1.8 |
| Microbial population (x105 CFU mLG-1) | ||||||||
| Coliform count | 0.25±0.53a | 0.91±0.25a | - |
10.0000±0.0000 |
0.2 |
0.3 |
0.45 |
1.25 |
| Total bacteria count | 0.85±0.26a | 1.26±0.23a | - |
- |
0.6 |
1.3 |
0.8 |
1.5 |
| E. coli count | 0.10±0.05ab | 0.33±0.17b | - |
0.0000±0.0000 |
0.1 |
0.2 |
0.1 |
0.7 |
| Salmonella count | 0.00±0.00a | 0.00±0.00a | - |
- |
- |
- |
- |
- |
| Vibrio cholera count | 0.00±0.00a | 0.00±0.00a | - |
- |
- |
- |
- |
- |
| S. aureus count | 0.16±0.07a | 0.32±0.12a | - |
- |
0.1 |
0.3 |
0.2 |
0.55 |
| Streptococcal count | 0.18 ±0.13a | 0.25±0.67a | - |
0.0000±0.0000 |
0.1 |
0.4 |
0.2 |
0.4 |
| Values represent mean±standard deviation (SD), values along the same row with different superscripts are significantly different at p<0.05 level, the means and standard deviations, minimum and maximum values for heavy and trace metal concentrations (mg L–1) and microbial populations (x105 CFU mL–1) in the water samples collected in the Eruwa farm settlement are presented in Table 3 | ||||||||
| Table 3: | Metal concentrations and microbial populations in the water samples from Eruwa farm settlement | |||
| Heavy and trace metal (mg L–1) |
Dry season | Wet season | WHO |
NSDWQ |
Dry season ------------------ |
Wet season ------------------ |
||
(2011) |
(2007) |
Min |
Max |
Min |
Max |
|||
| Lead | 0.0025±0.0050a | 0.0150±0.0058b | 0.0100±0.0000 |
0.0100±0.0000 |
0.001 |
0.001 |
0.01 |
0.02 |
| Cadmium | 0.0003±0.0050a | 0.0175±0.0150a | 0.0030±0.0000 |
0.0030±0.0000 |
0.001 |
0.001 |
0.01 |
0.04 |
| Zinc | 0.2575±0.1725a | 1.1525±0.0822b | - |
3.0000±0.0000 |
0.32 |
0.36 |
1.06 |
1.24 |
| Manganese | 0.6500±0.0904a | 0.9900±0.4044b | 0.4000±0.0000 |
0.2000±0.0000 |
0.01 |
0.2 |
0.59 |
1.4 |
| Copper | 0.0050±0.0058a | 0.9875±0.1443b | 2.0000±0.0000 |
1.0000±0.0000 |
0.01 |
0.01 |
0.81 |
1.11 |
| Chromium | 0.0002±0.0004a | 0.0000±0.0000a | 0.0500±0.0000 |
0.0500±0.0000 |
- |
- |
- |
- |
| Aluminium | Not detected | Not detected | 0.1000±0.0000 |
0.2000±0.0000 |
- |
- |
- |
- |
| Arsenic | Not detected | Not detected | 0.0100±0.0000 |
0.0100±0.0000 |
- |
- |
- |
- |
| Mercury | Not detected | Not detected | 0.0060±0.0000 |
0.0010±0.0000 |
- |
- |
- |
- |
| Boron | Not detected | Not detected | 2.4000±0.0000 |
- |
- |
- |
- |
- |
| Iron | 0.1750±0.0420a | 1.2900±0.1388b | 0.1000±0.0000 |
0.2000±0.0000 |
0.13 |
0.23 |
1.11 |
1.43 |
| Microbial population (x105 CFU mL–1) | ||||||||
| Coliform count | 0.33±0.10a | 0.90±0.30a | 10.00±0.00 |
10.0000±0.0000 |
0.2 |
0.4 |
0.6 |
1.25 |
| Total bacteria count | 1.20±0.37a | 0.79±0.25a | - |
- |
0.7 |
1.6 |
0.5 |
1 |
| E. coli count | 0.15±0.06a | 0.08±0.10b | 0.00±0.00 |
0.0000±0.0000 |
0.1 |
0.2 |
0.1 |
0.2 |
| Salmonella count | 0.00±0.00a | 0.00±0.00a | - |
- |
- |
- |
- |
- |
| Vibrio cholera count | 0.00±0.00a | 0.00±0.00a | - |
- |
- |
- |
- |
- |
| S. aureus count | 0.33±0.05a | 0.20±0.08a | - |
- |
0.3 |
0.4 |
0.1 |
0.3 |
| Streptococcal count | 0.30±0.08a | 0.15±0.06a | 0.00±0.00 |
0.0000±0.0000 |
0.2 |
0.4 |
0.1 |
0.2 |
| Values represent mean±standard deviation (SD) and values along the same row with different superscripts are significantly different at p<0.05 level | ||||||||
| Table 4: | Factor loadings for heavy and trace metals as well as microbial populations in the water samples from Ajegunle farm settlement | |||
| Variable | Principal component -------------------------------------------------------------------------------------------------------- |
|||
1 |
2 |
3 |
4 |
|
| Lead | -0.415 |
0.094 |
0.777 |
0.1 |
| Cadmium | -0.147 |
0.035 |
0.852 |
0.026 |
| Zinc | 0.862 |
-0.189 |
-0.154 |
-0.276 |
| Manganese | 0.896 |
-0.132 |
-0.115 |
-0.241 |
| Copper | 0.917 |
-0.205 |
-0.213 |
-0.156 |
| Iron | 0.89 |
0.071 |
-0.152 |
0.191 |
| Chromium | -0.39 |
0.168 |
-0.312 |
0.821 |
| Aluminum | -0.008 |
-0.157 |
0.404 |
0.864 |
| Arsenic | 0.042 |
0.8 |
0.443 |
-0.172 |
| Mercury | -0.172 |
0.968 |
-0.052 |
0.059 |
| Boron | -0.172 |
0.968 |
-0.052 |
0.059 |
| Initial eigenvalues | 4.608 |
2.311 |
1.616 |
1.287 |
| Variance (%) | 41.893 |
21.012 |
14.687 |
11.701 |
| Cumulative (%) | 41.893 |
62.905 |
77.592 |
89.293 |
| Coliform count | 0.11 |
0.833 |
||
| Total bacteria count | 0.819 |
0.092 |
||
| Escherichia coli count | 0.75 |
-0.361 |
||
| Staphylococcus aureus count | 0.057 |
-0.649 |
||
| Streptococcal count | 0.796 |
0.15 |
||
| Initial eigenvalues | 1.883 |
1.276 |
||
| Variance (%) | 37.663 |
25.522 |
||
| Cumulative (%) | 37.663 |
63.185 |
||
| Extraction method: principal component analysisa, Rotation method: Varimax with Kaiser normalization and a2 components extracted | ||||
| Table 5: | Factor loadings for heavy and trace metals as well as microbial populations in the water samples from Akufo farm settlement | |||
| Variable | Principal component ------------------------------------------------------------------------------- |
|
1 |
2 |
|
| Lead | 0.037 |
0.891 |
| Cadmium | 0.347 |
0.782 |
| Zinc | 0.94 |
0.276 |
| Manganese | 0.96 |
0.238 |
| Copper | 0.966 |
0.045 |
| Iron | 0.952 |
0.213 |
| Initial eigenvalues | 4.182 |
1.171 |
| Variance (%) | 69.696 |
19.512 |
| Cumulative (%) | 69.696 |
89.208 |
| Coliform count | 0.827 |
|
| Total bacteria count | 0.833 |
|
| Escherichia coli count | 0.69 |
|
| Staphylococcus aureus count | 0.73 |
|
| Streptococcal count | 0.54 |
|
| Initial eigenvalues | 2.677 |
|
| Variance (%) | 53.541 |
|
| Cumulative (%) | 53.541 |
|
| Extraction method: principal component analysisa and a1 component extracted | ||
| Table 6: | Factor loadings for heavy and trace metals as well as microbial populations in the water samples from Eruwa farm settlement | |||
Principal component ------------------------------------------------------------------------------- |
||
| Variable | 1 |
2 |
| Lead | 0.932 |
|
| Cadmium | 0.734 |
|
| Zinc | 0.971 |
|
| Manganese | 0.835 |
|
| Copper | 0.981 |
|
| Iron | 0.986 |
|
| Initial eigenvalue | 4.981 |
|
| Variance (%) | 83.011 |
|
| Cumulative (%) | 83.011 |
|
| Coliform count | -0.824 |
-0.47 |
| Total bacteria count | 0.814 |
0.055 |
| Escherichia coli count | 0.02 |
0.993 |
| Staphylococcus aureus count | 0.948 |
-0.12 |
| Streptococcal count | 0.714 |
0.487 |
| Initial eigenvalues | 3.048 |
1.166 |
| Variance (%) | 60.966 |
23.318 |
| Cumulative (%) | 60.966 |
84.284 |
| Extraction method: principal component analysisa, Rotation method: Varimax with Kaiser normalization and a2 components extracted | ||
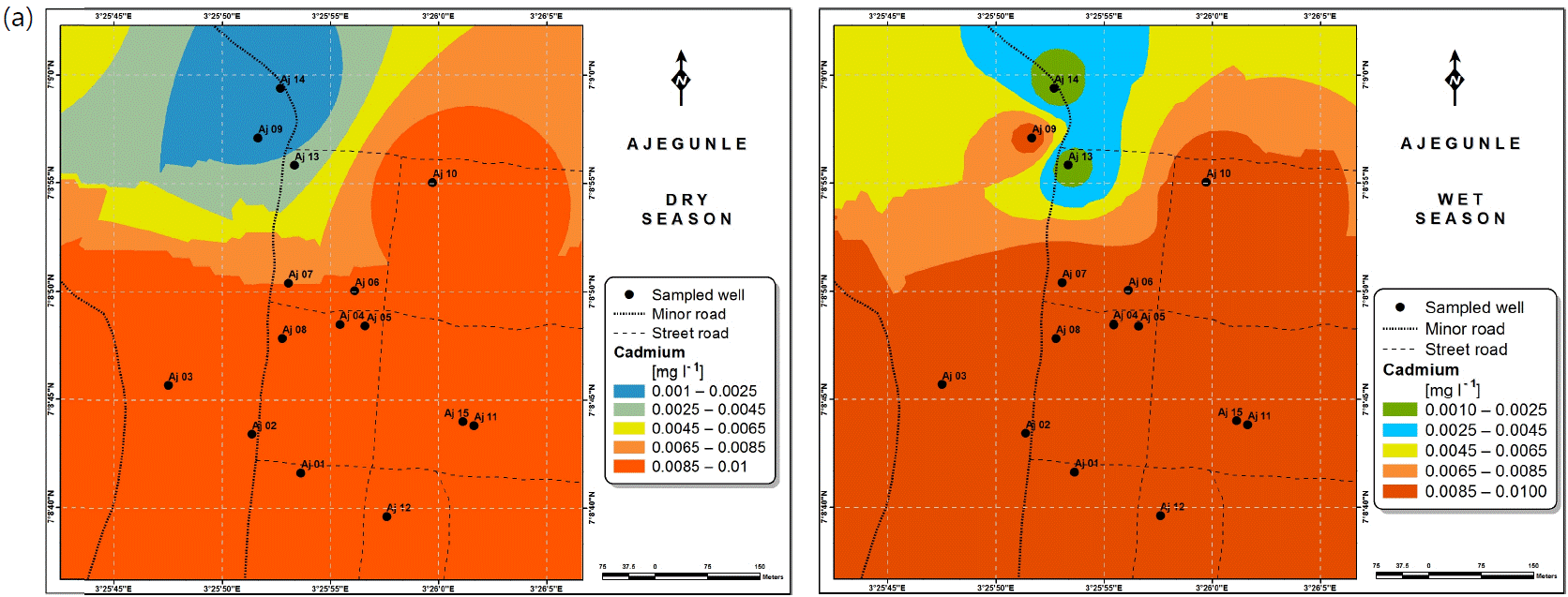
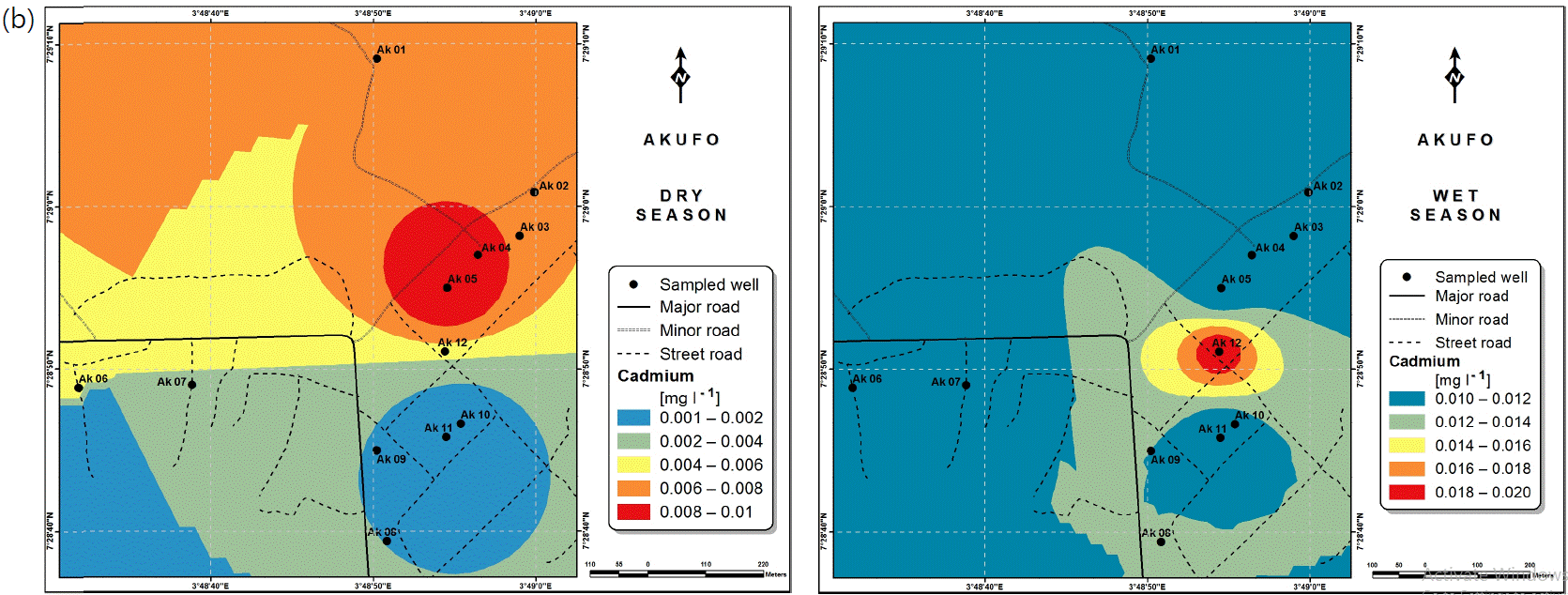
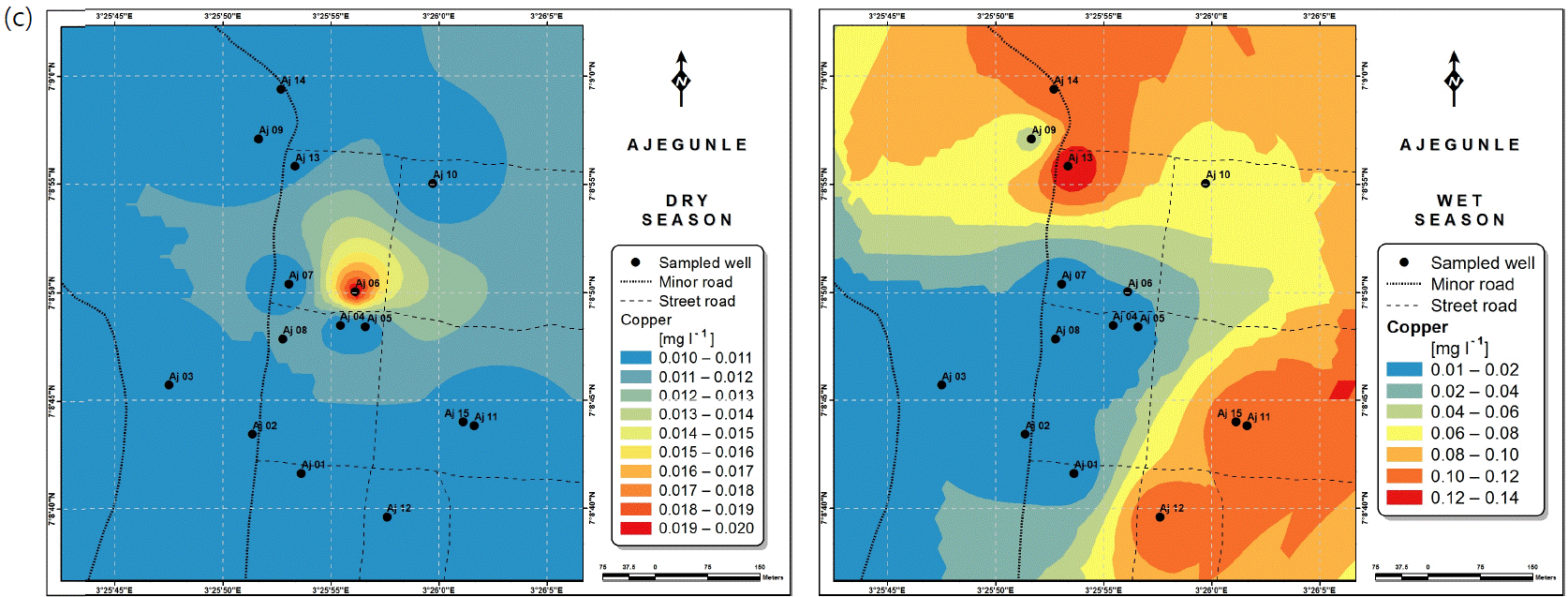
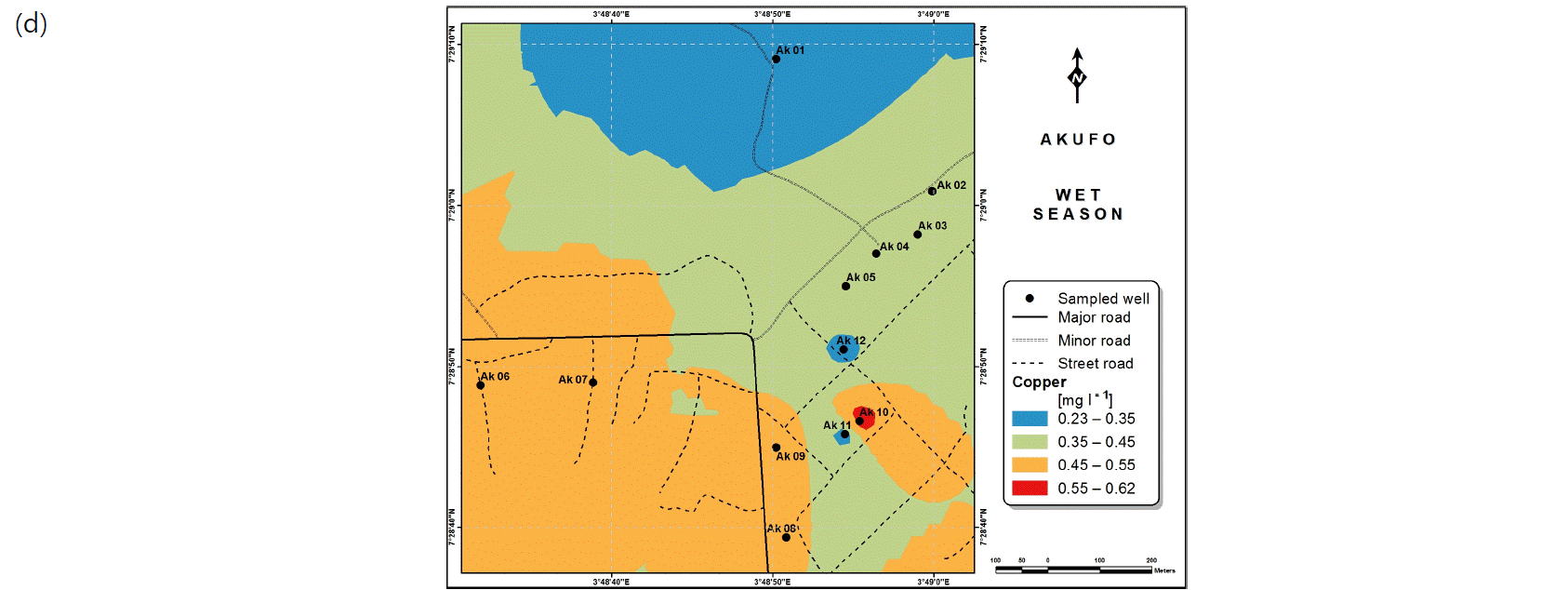
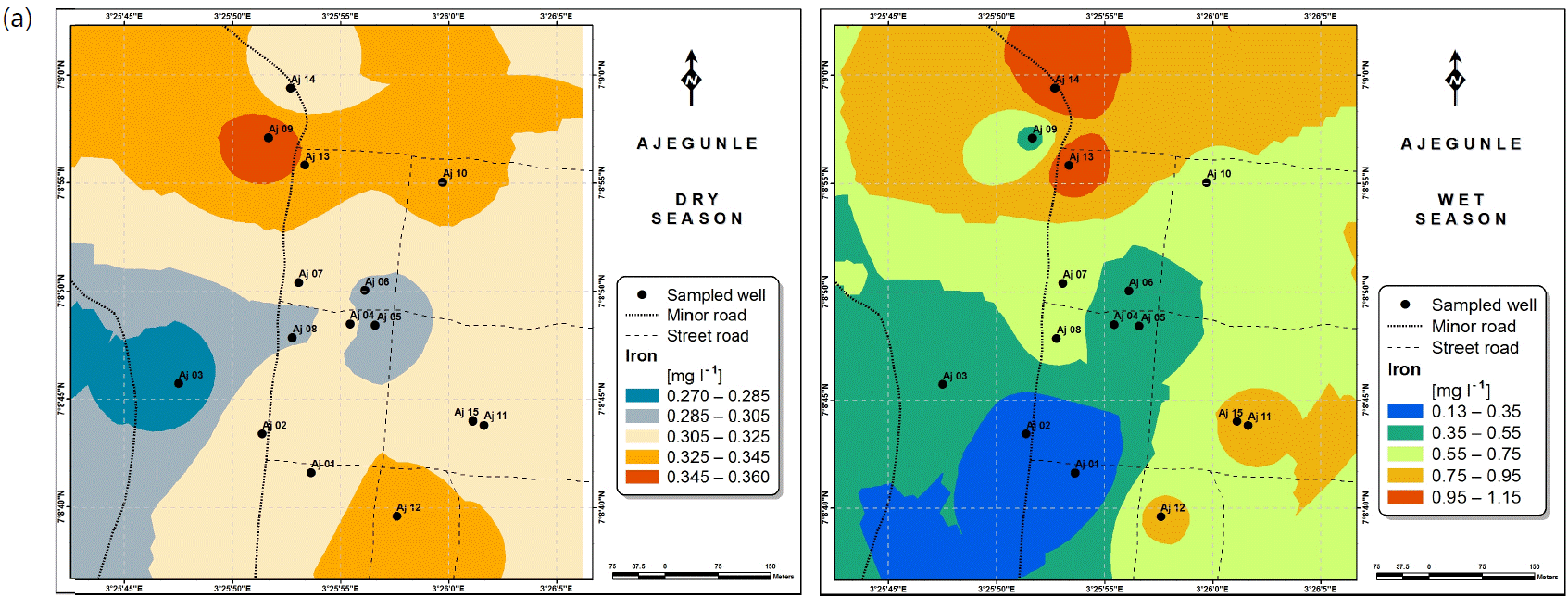
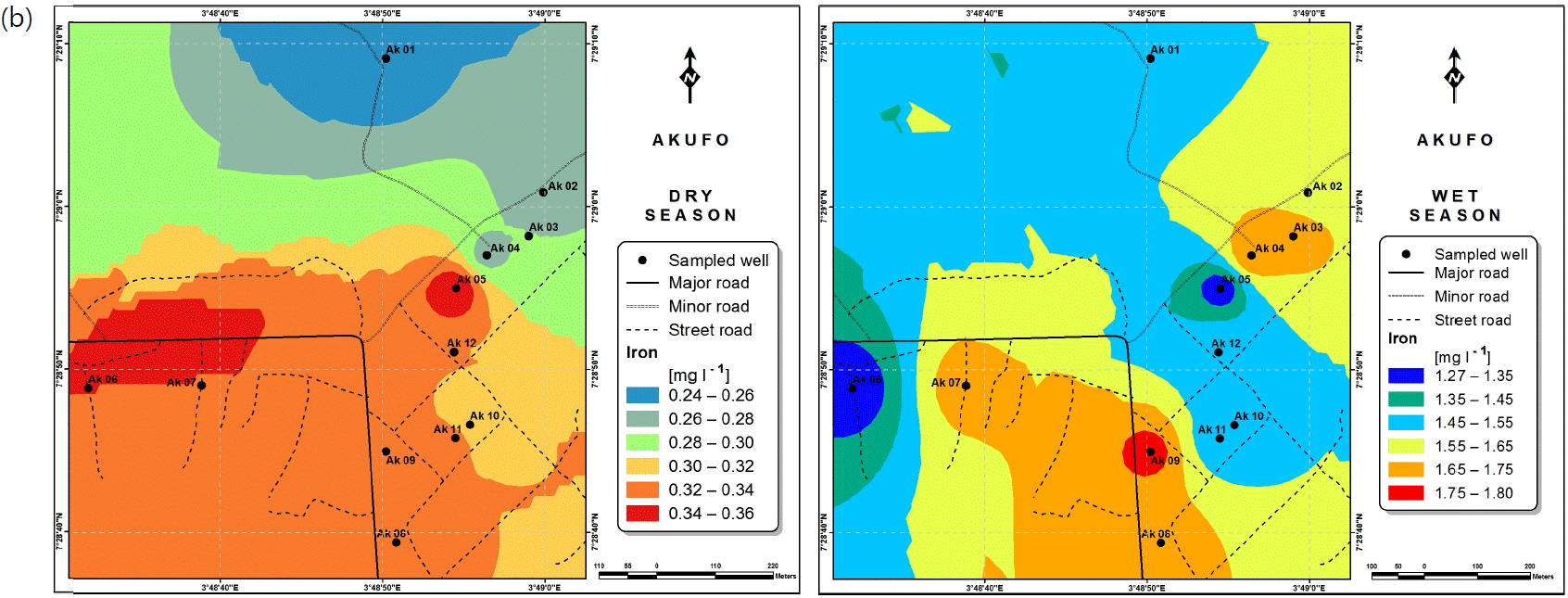
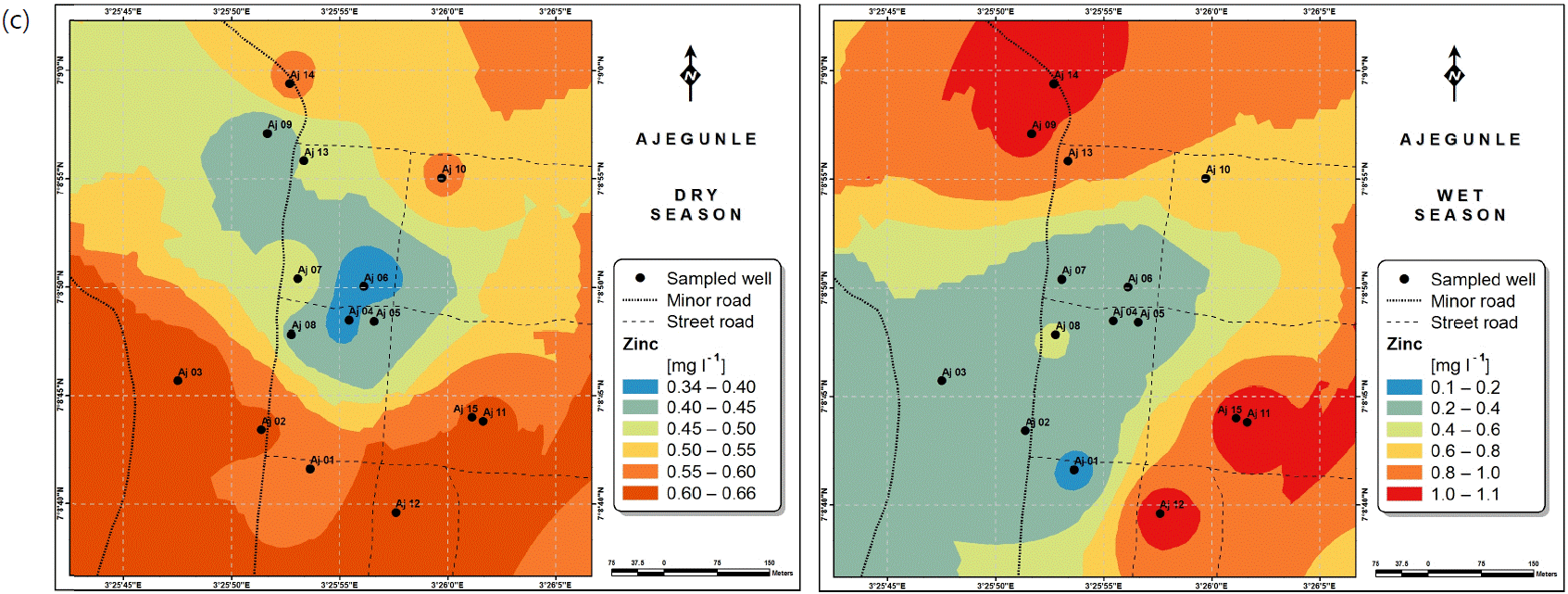
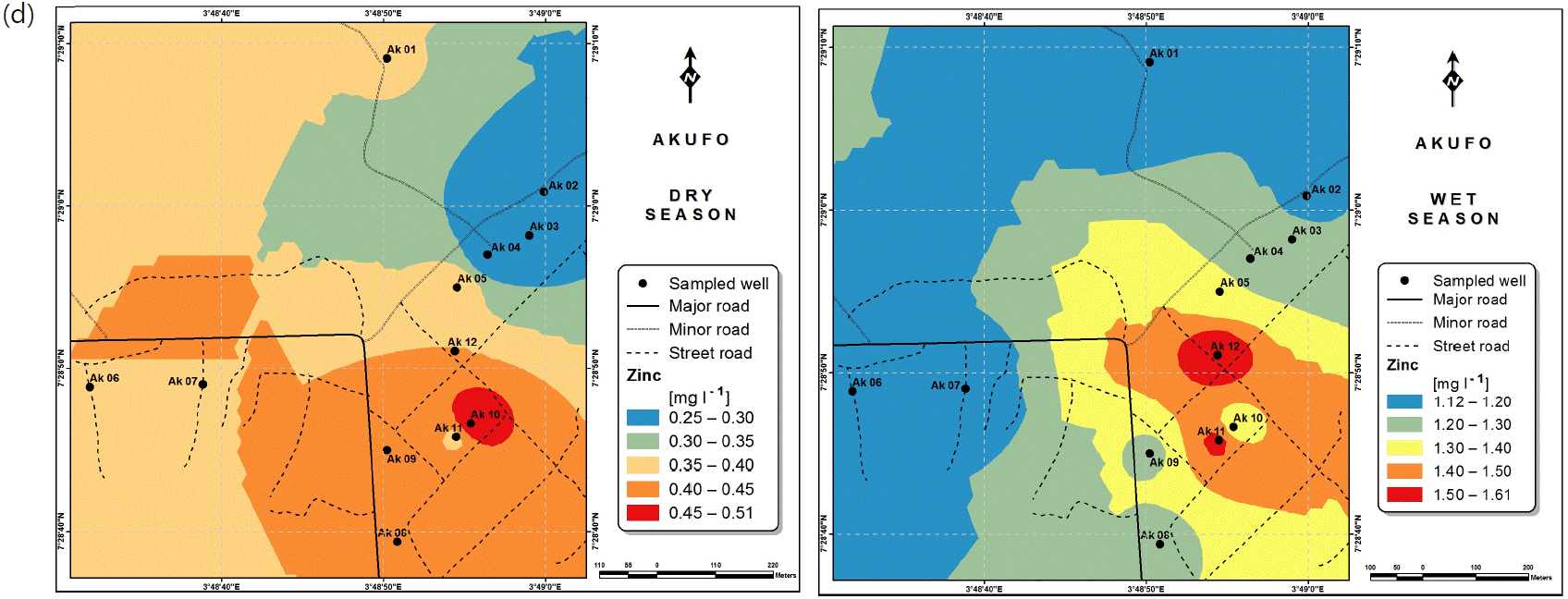
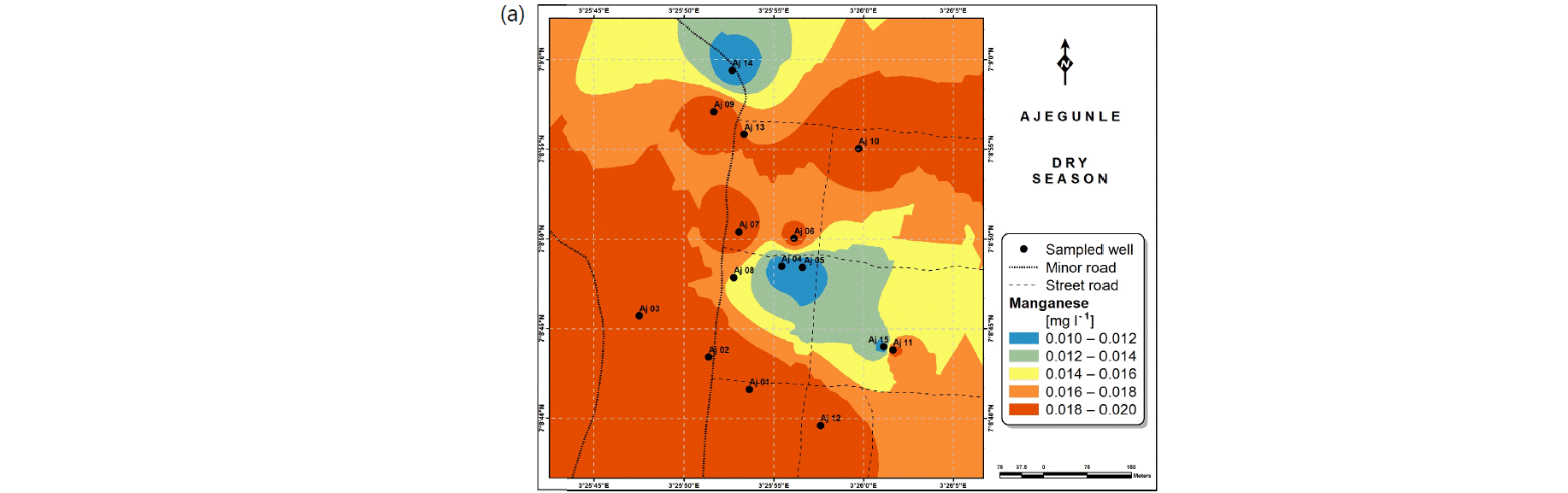
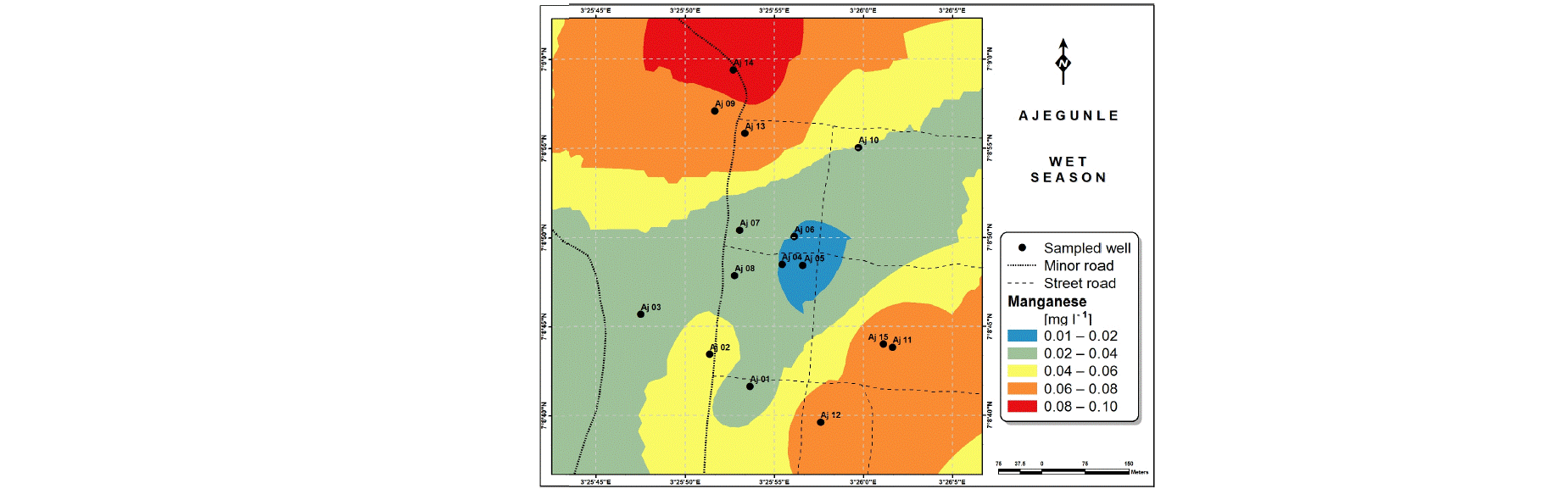
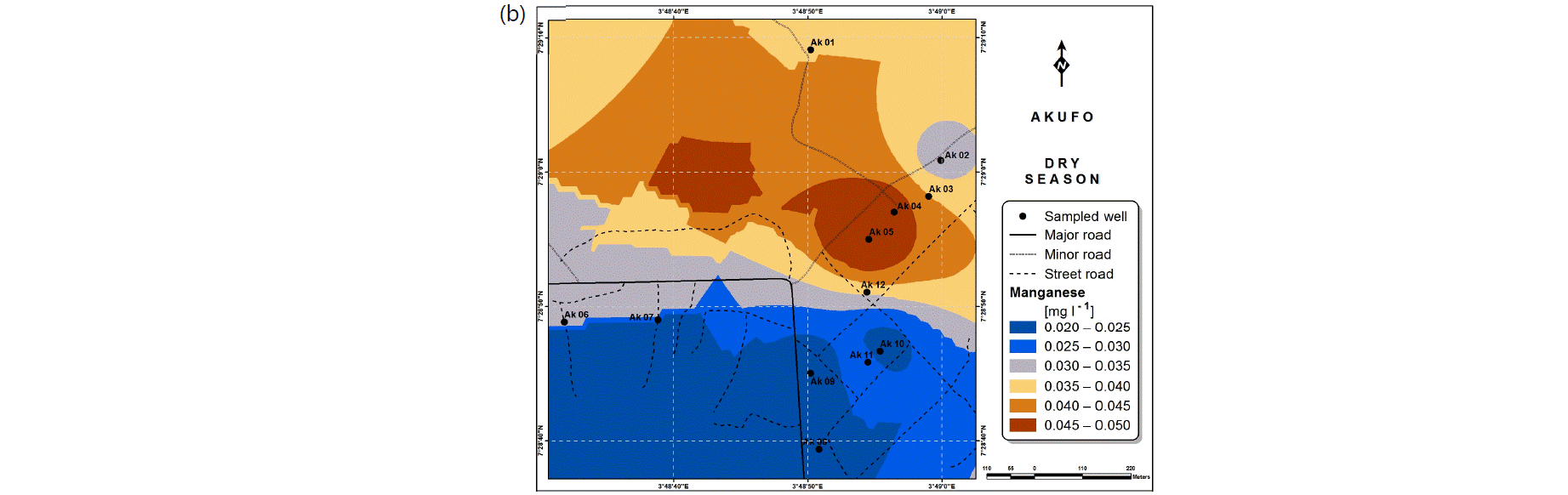
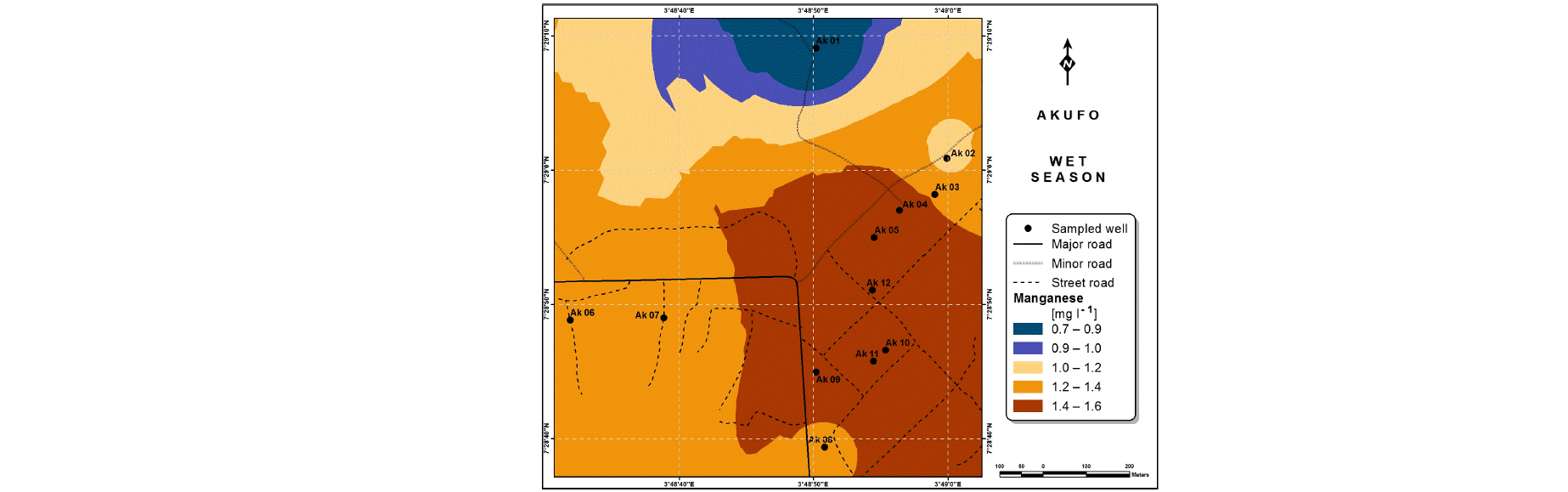
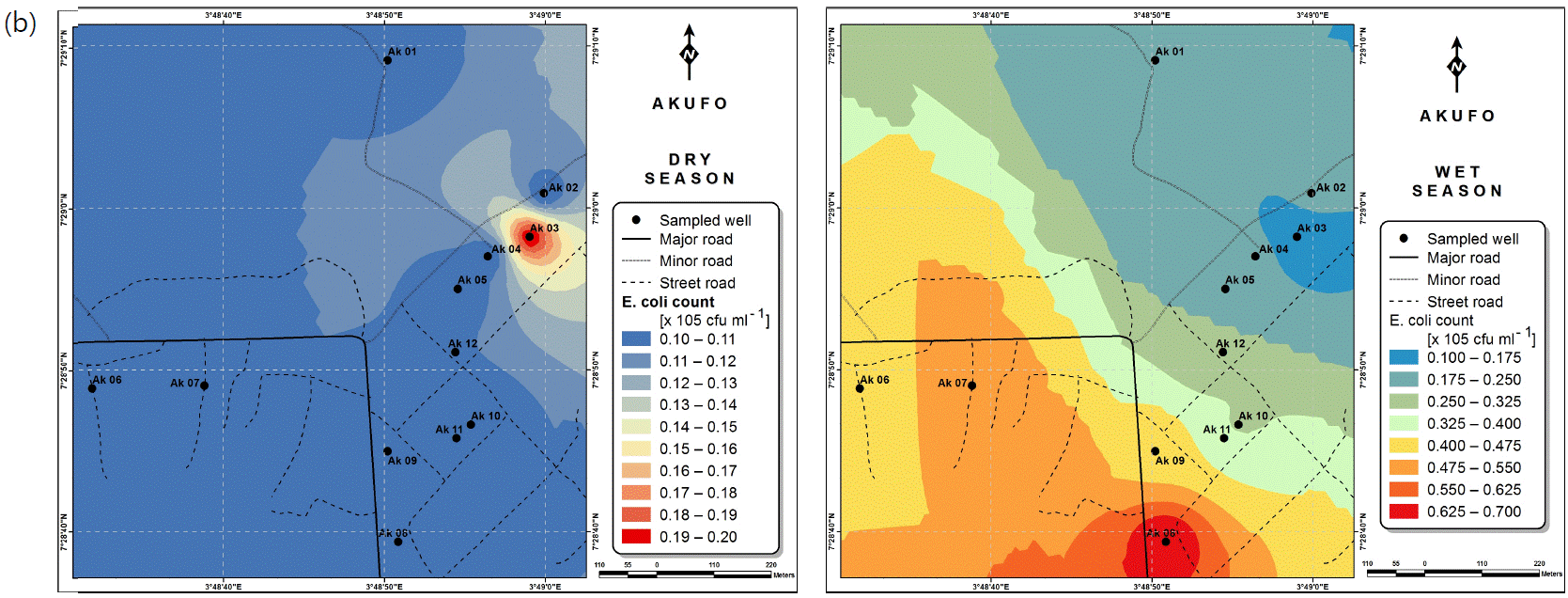
Spatial variabilities: The spatial distributions of selected metals for Ajegunle and Akufo farm settlements during the dry and wet seasons respectively are presented as follows: Cadmium in Fig. 3a-b while Copper in Fig. 3c-d, Iron in Fig. 4a-b while Zinc in Fig. 4c-d and Manganese in Fig. 5a-b.
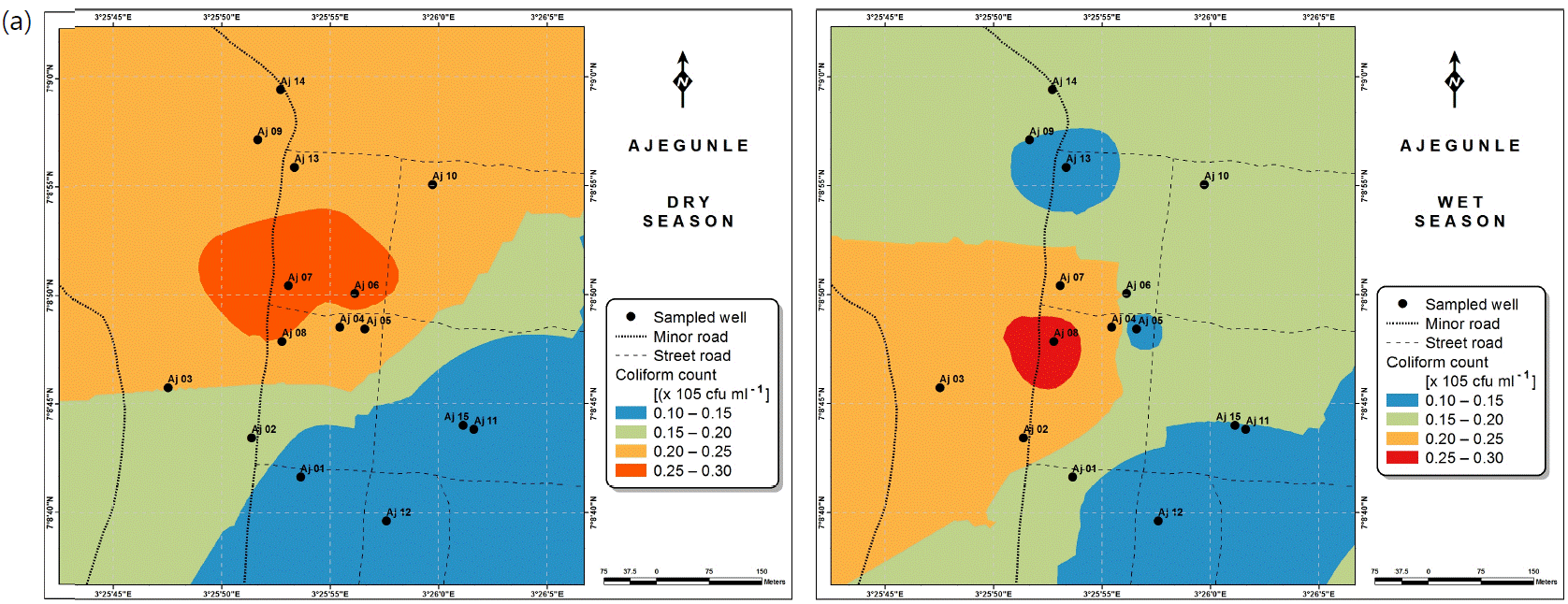
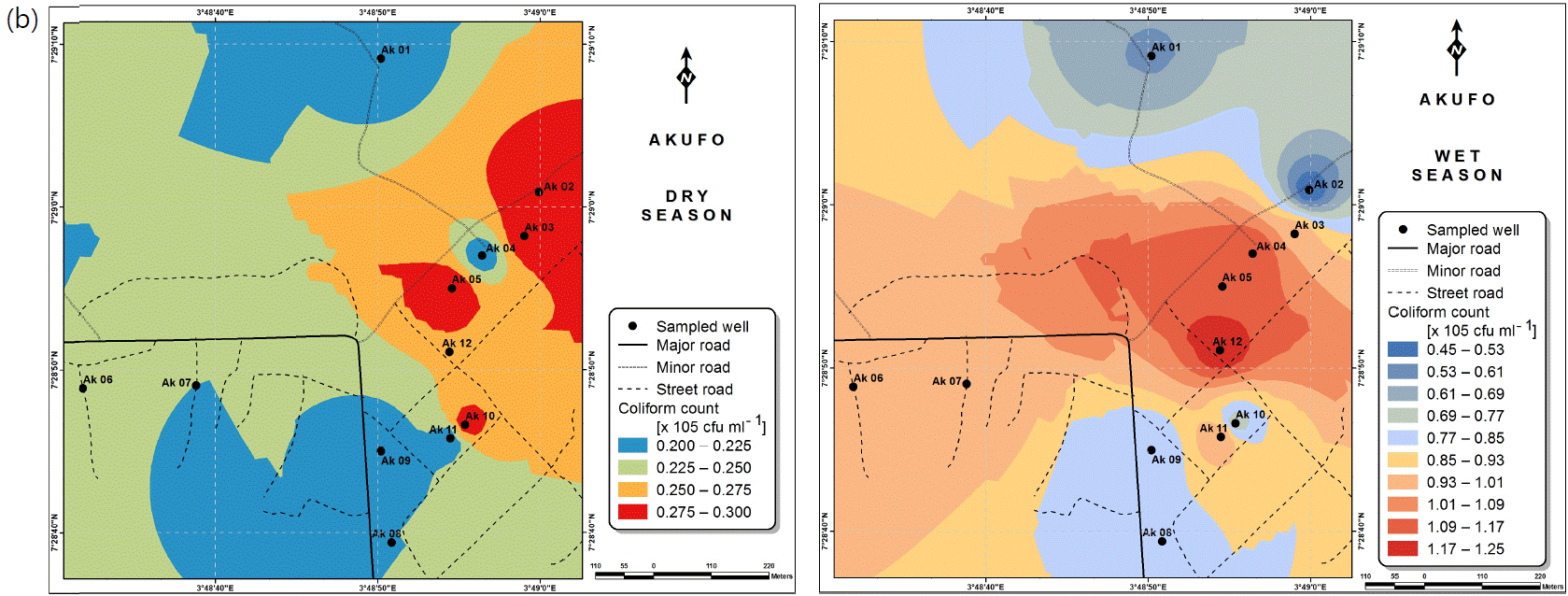
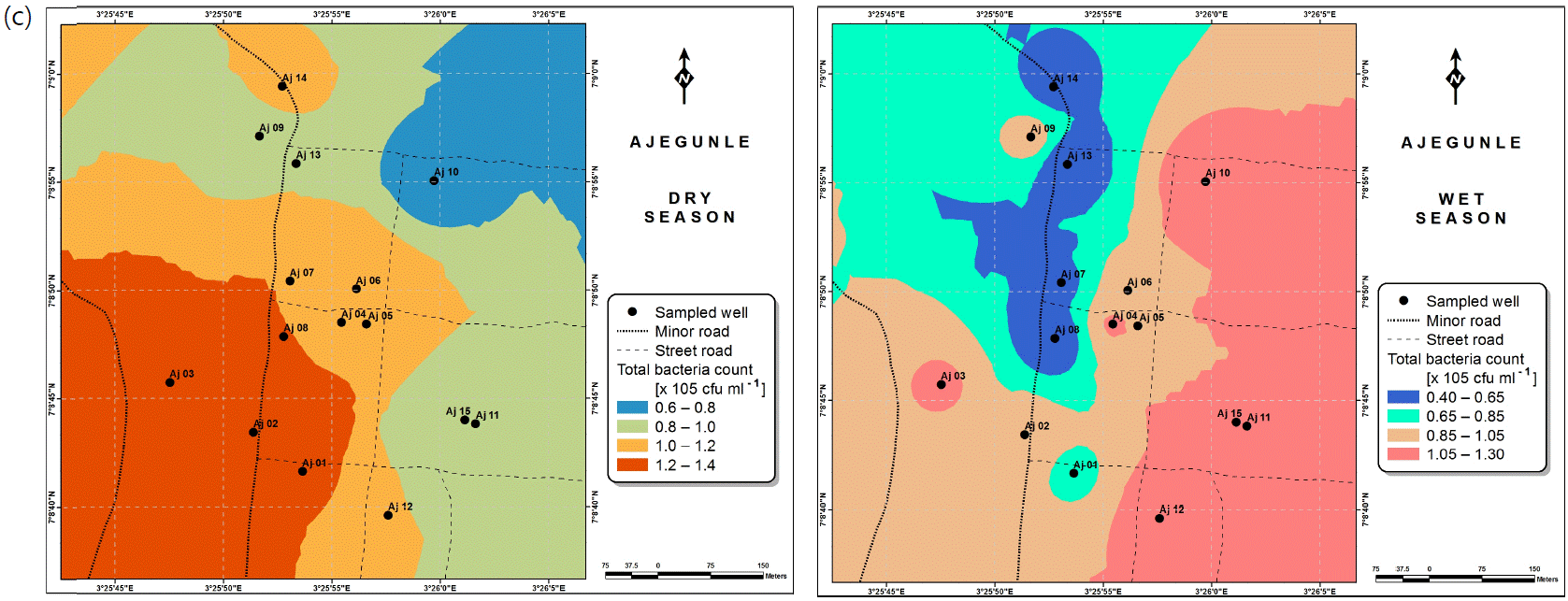
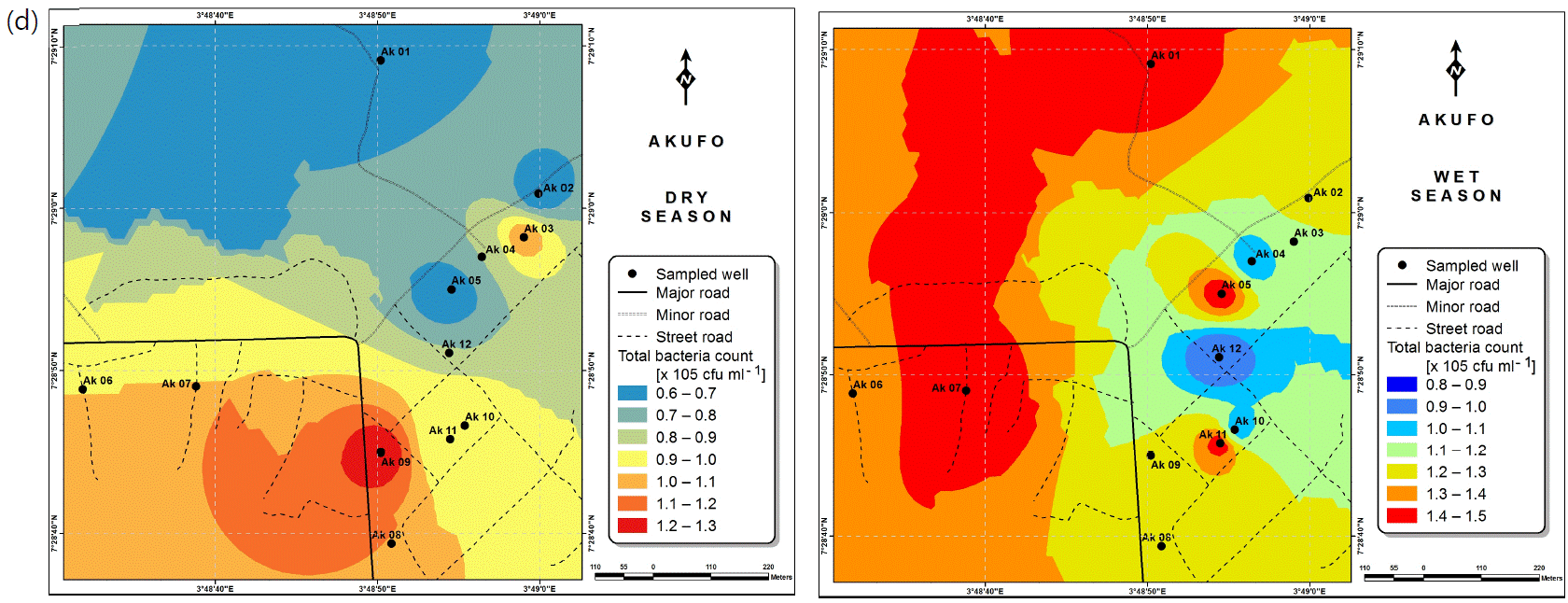
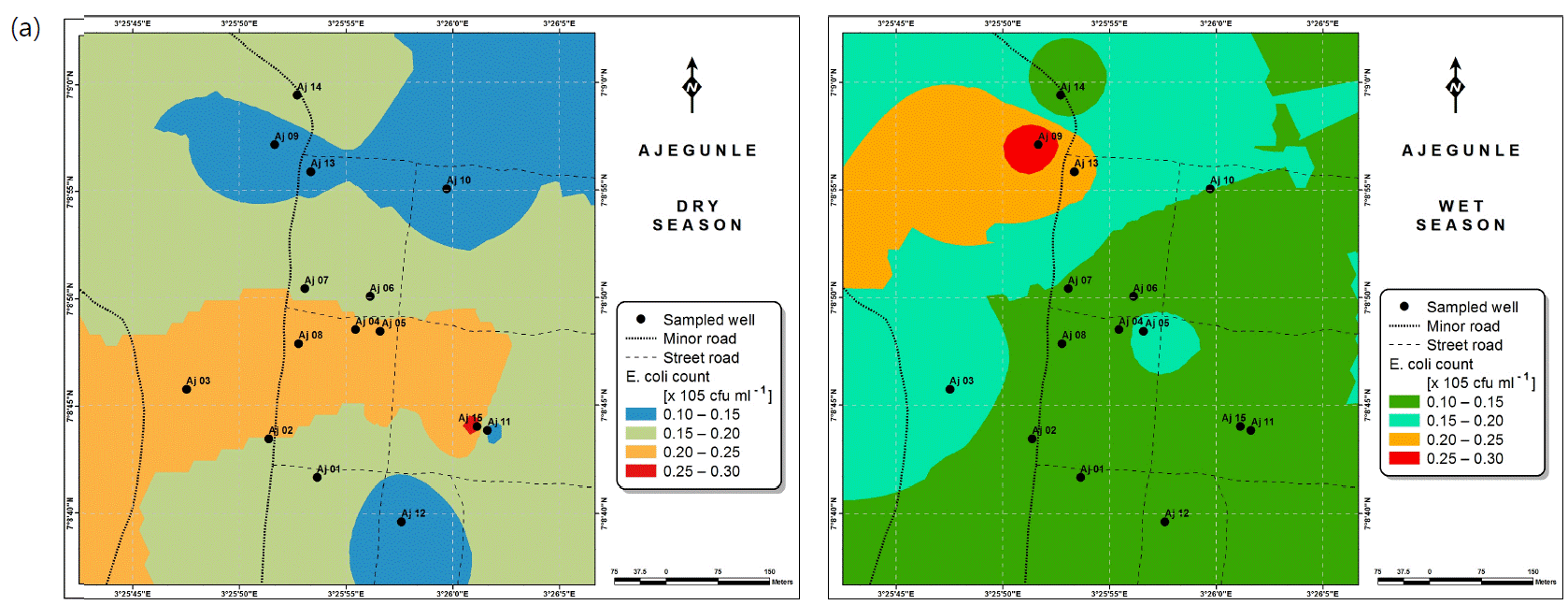
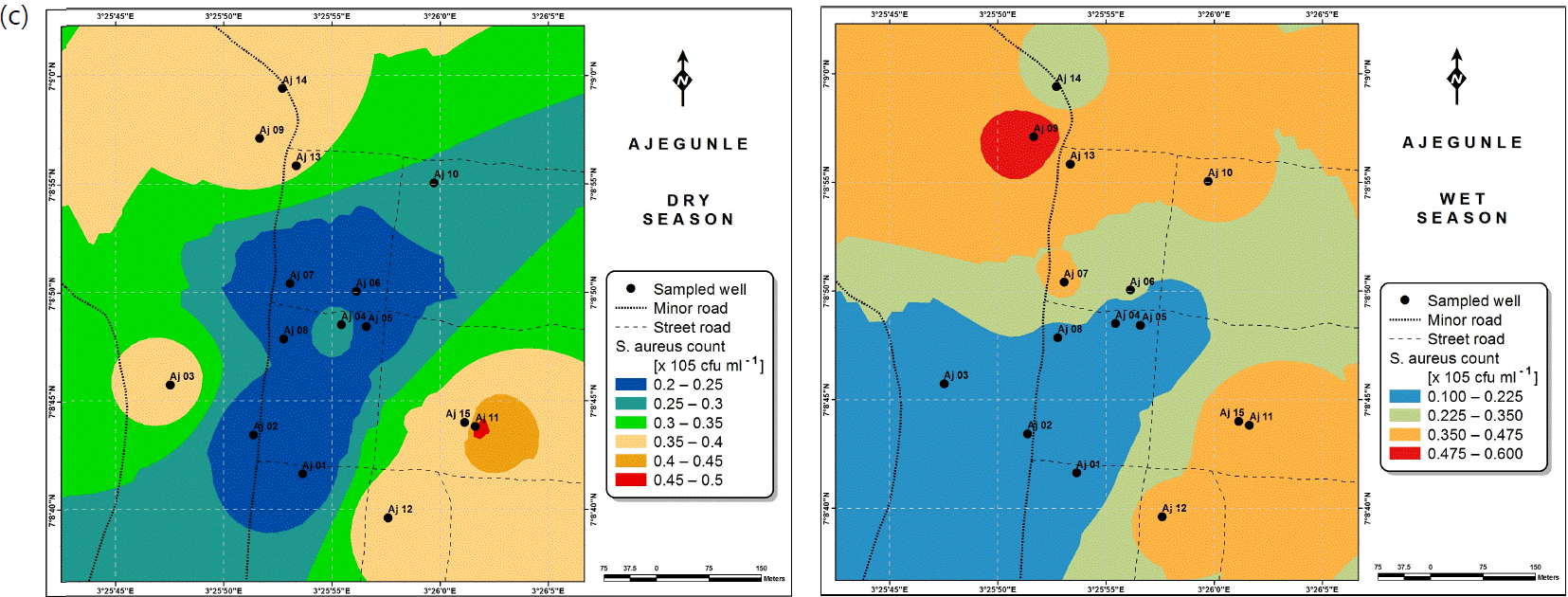
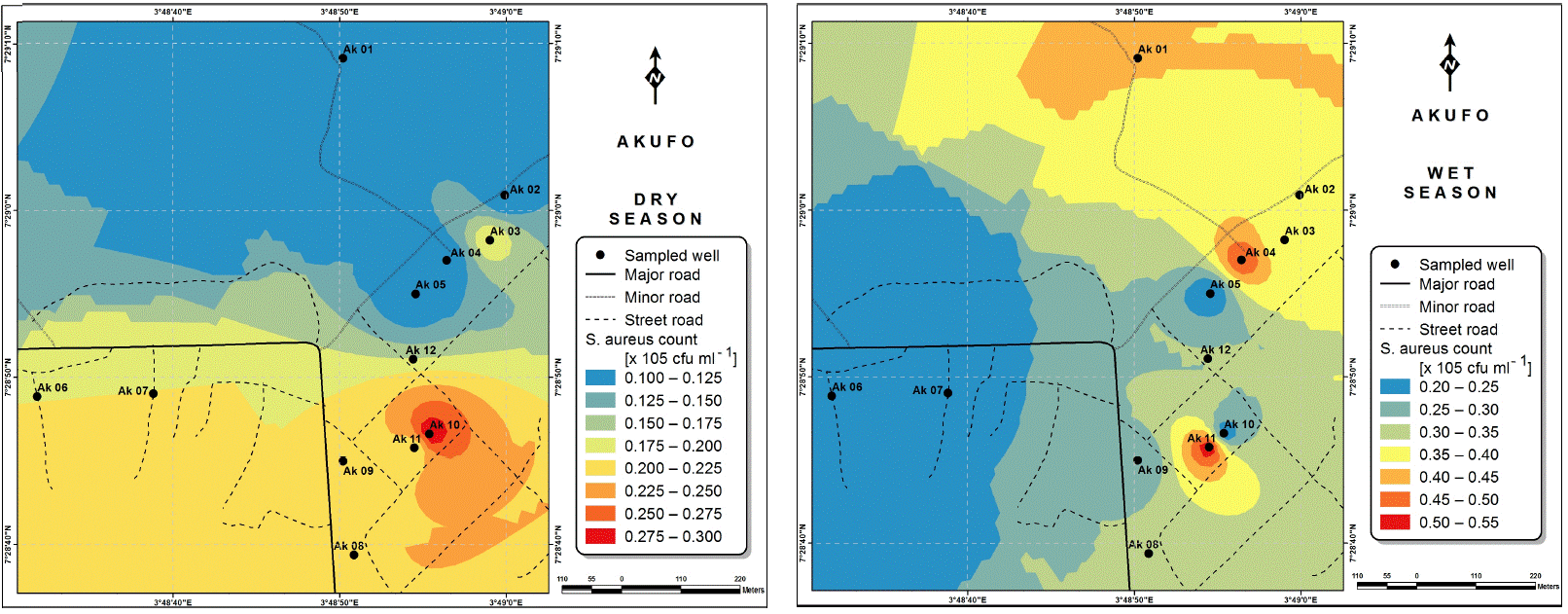
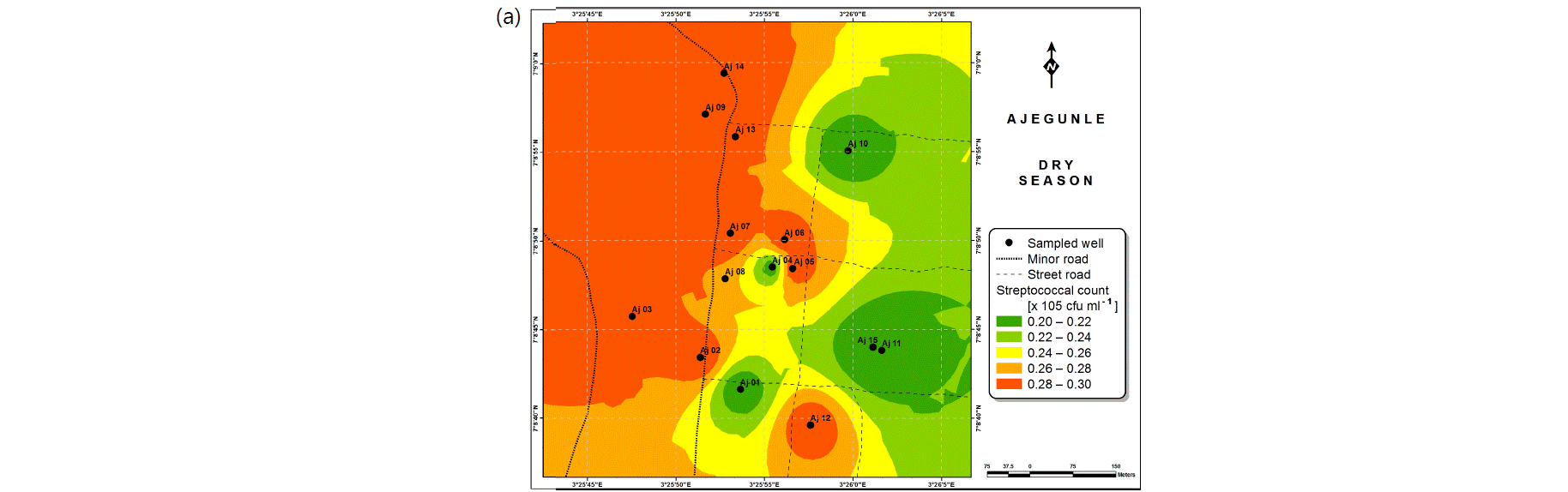
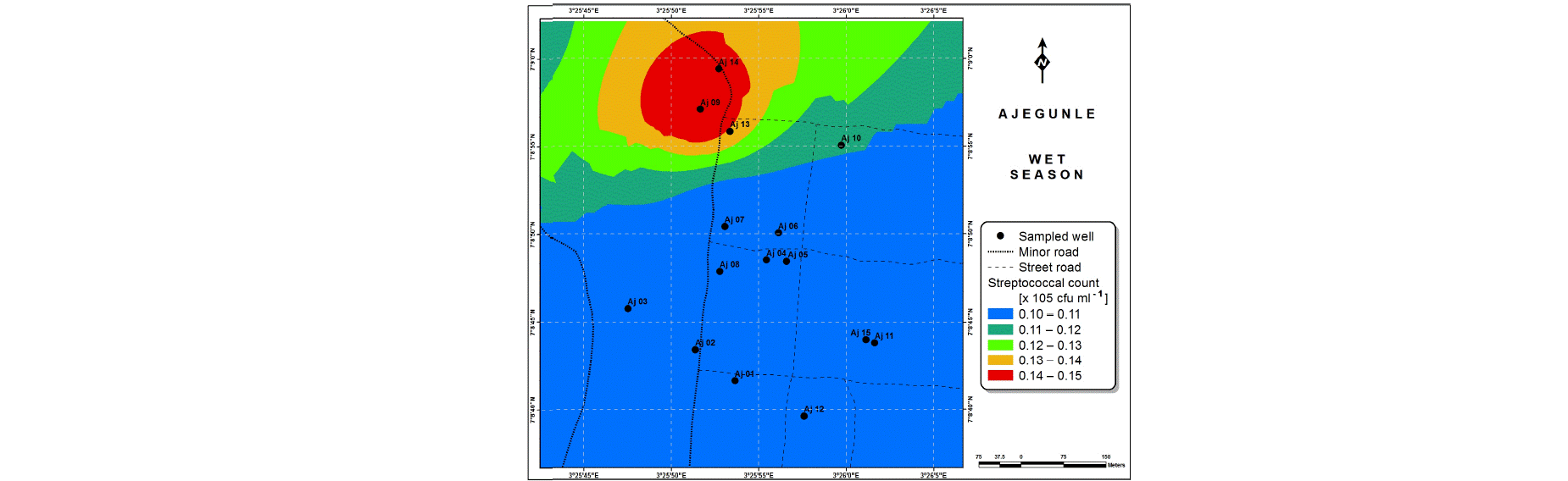
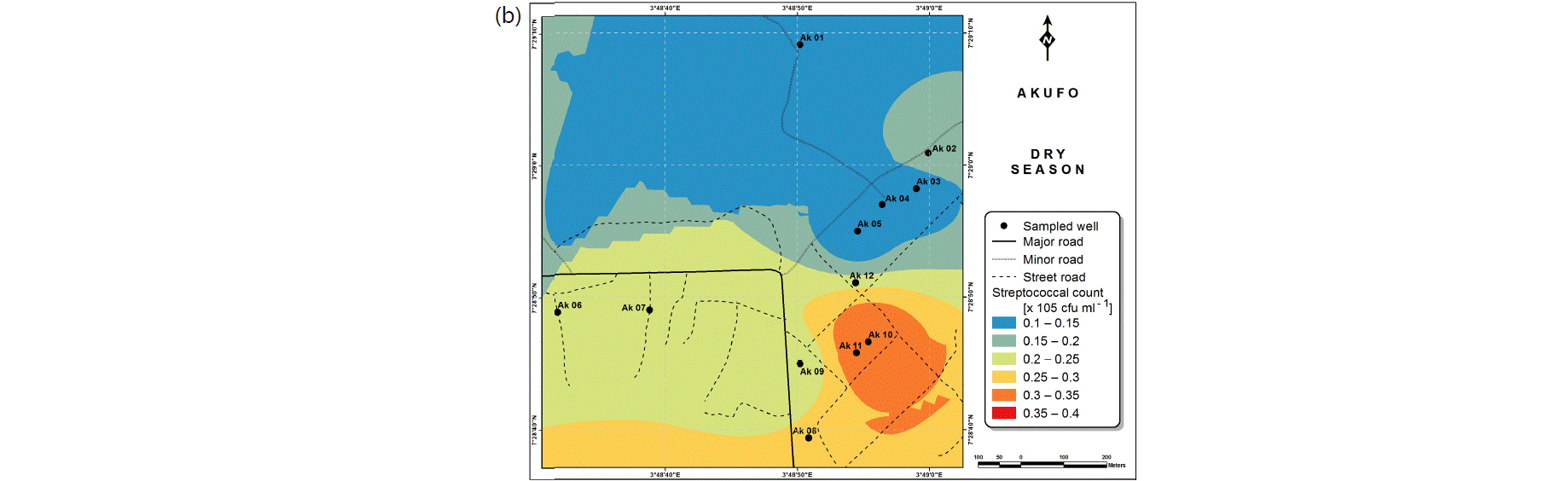
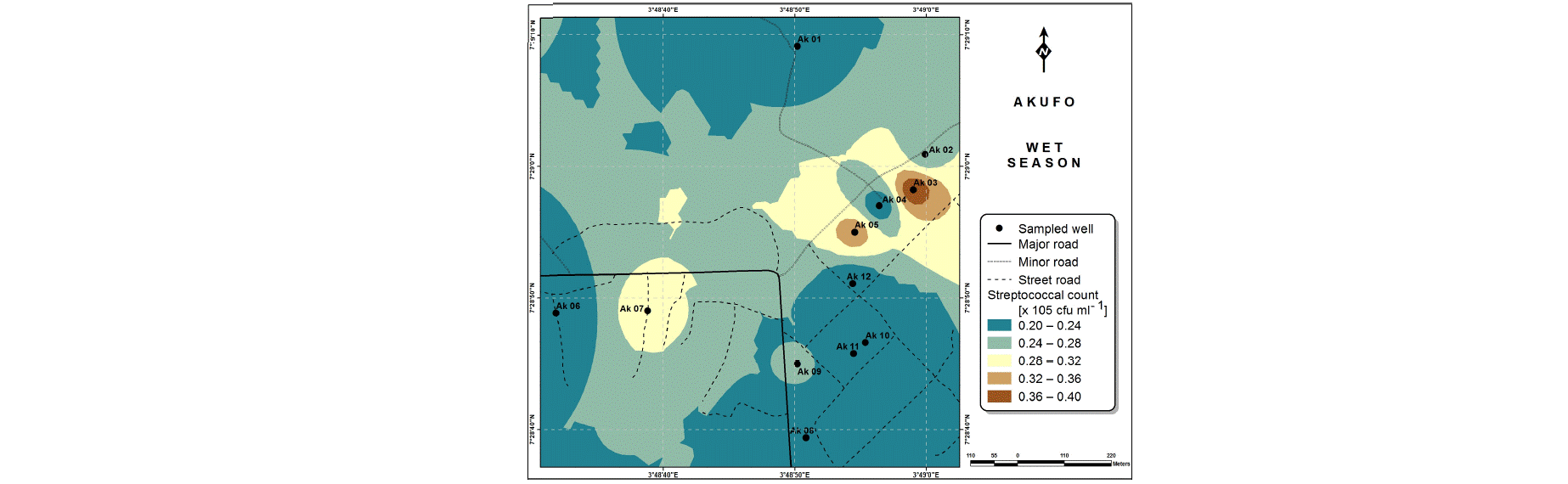
Also, the spatial distributions of selected microbial populations for Ajegunle and Akufo farm settlements during the dry and wet seasons, respectively are presented as follows: Coliform counts in Fig. 6a-b while total bacteria counts in Fig. 6c-d, E. coli counts in Fig. 7a-b while S. aureus counts in Fig. 7c-d and Streptococcal counts in Fig. 8a-b.
|
|
|
|
|
|
DISCUSSION
For the water samples collected from the Eruwa farm settlement, the high levels of Copper in the wet season were likely due to agricultural activities. As shown via this study, the groundwater from the three farm settlements should be monitored and treated for cadmium, iron and manganese contaminations.
Heavy metals are usually either dissolved in water or occur as colloids and/or particles31. They occur in water bodies naturally as eroded minerals within sediments, leached ore deposits, etc., or originate from anthropogenic sources such as solid waste disposal, as well as industrial, agricultural and domestic effluents32. Dissociations from bedrocks through which groundwater flows also contribute to metal concentrations in it33. Levels of Copper above 1 mg L–1 in water have been noted to tend to cause astringent tastes34.
As revealed via this study, Arsenic poses no threat to the water within the three farm settlements. Kayode et al.35 discovered high concentrations of Arsenic and Cadmium in some parts of Ogun State, Nigeria. High concentrations of Cadmium can negatively affect seed germination and plant development as well as generate oxidative damages36,37. Elevated Cadmium concentrations in humans can contribute to high blood pressure and kidney damage as well as the destruction of red blood cells and testicular tissues. Cadmium, due to their chemical similarity, may replace Zinc in some enzymes and thus alter the stereo-structure of the enzymes, impairing their catalytic activities35,38.
The major sources of Manganese are ores, rocks, fertilizers, steel production, pesticides and battery charging34. Studies have associated cognitive behavioral problems in children with Manganese concentration in drinking water39,40.
Iron is common in rural groundwater supplies with concentration levels between 0 and 50 mg L–1. According to Al Maliki et al.37, it is not toxic to plants where the soil is aerated but can enhance processes that lead to soil acidification and loss of phosphorus, which is an important element for plant well-being. Iron can accumulate on plant leaves and/or fruits as a result of long-term irrigation with water containing Iron in high concentrations, which in turn can reduce the quality of crop production37,41. No serious health implications have been reported in humans42. It has however been noted that in high concentrations, Iron may produce adverse neurological effects43. Iron-mediated oxidative damage to the mitochondrial genome may result from long-term iron contamination, eventually adversely affecting functionality40,44.
The concentrations of Iron and Manganese in this study follow a similar trend to what Emenike et al.45 observed, Iron and Manganese usually exist together in water, the concentration of Iron being always higher than that of Manganese because it is more abundant in the earth crust.
As excess heavy and trace metal intake by humans through the food chain (or agriculture) has been reported to be potentially dangerous in many countries1, it is recommended that the water from the three farm settlements be monitored and treated for cadmium, iron and manganese contaminations. This is to protect the consumers of agricultural produce from the potential dangers of consuming the metals in higher quantities than permissible. Also, crops that are resistant to, or require high concentrations of these metals are recommended for cultivation within the farm settlements.
Several studies have shown that microbial pathogens, such as Salmonella, E. coli, S. faecalis and enteroviruses are relatively stable in underground water46-49, which is a major source of water for agriculture within the farm settlements.
High coliform counts appear to be characteristic of rural groundwater quality in Nigeria50. Meanwhile for the three farm settlements, coliform counts for both dry and wet seasons considered were revealed to be safe. However, E. coli and Streptococcal counts for the two seasons suggest the need for appropriate treatment before use. This likely resulted mostly from livestock wastes in the farm settlements, since livestock agriculture is a major occupation of the settlers. It could as well be suspected that these contaminants infiltrated from human and animal wastes into the hand-dug wells51. Escherichia coli is regarded as the most sensitive indicator of faecal pollution and its presence in a water sample is a major health concern which calls for remedial attention51. Its presence also indicates that other enteric pathogens may be present52. Streptococcus faecalis, Staphylococcus aureus and Bacillus sp., have been implicated to be possibly responsible for gastro-intestinal disorders53. Salmonella and vibrio cholera were not found in any of the samples examined from the farm settlements.
Consumption of contaminated water has been known to result in diseases such as diarrhea, meningitis, acute renal failure, urinary tract infections and haemolytic anaemia, or indirectly from contaminated agricultural produce. Meanwhile, Terzieva and McFeters54 as well as Pandey et al.49 noted that controlling pathogen contamination from livestock/wildlife can be challenging.
The principal component analysis (PCA) is a multivariate statistical technique that can use mutual correlation coefficients to relate variables to principal components or factors. When used in hydrochemistry, these may be interpreted based on specific or multiple hydrochemical processes like mineralization, lithology and environmental processes8,55.
The PCA extracted 4 components for Ajegunle farm settlement. The PC 1 resulted very likely from the weathering of bedrock materials, in line with Kwami et al.56. The PC 2 was likely from the leaching of agricultural wastes and/or chemicals. The PC 3 was likely due to leachate from fertilizers/agricultural wastes or run-off while PC 4 resulted from agricultural activities. Fertilizers used for agriculture are well-known sources of cadmium and copper57. For the Akufo farm settlement, PCA produced 2 components. The PC 1 resulted very likely from weathering of bedrock materials, similar to apportionment made by Shrestha et al.58, while PC 2 was likely due to leachate from fertilizers/agricultural wastes or run-off57,59. Meanwhile, for the Eruwa farm settlement, the only PC extracted was proposed to have been from weathering of bedrock materials and leachate from fertilizers/agricultural wastes or run-off, in line with the findings of Elumalai et al.57 The farm settlements have a long history of over 60 years of cultivation of staple crops, use of pesticides, fertilizers, other veterinary drugs and agrochemicals.
Heavy and trace metal concentrations/distributions have been reported to vary seasonally60,61. This study revealed higher concentrations of most of the metals during the wet season than during the dry. Agricultural activities form a major source of metal and microbial contamination in the water within the three farm settlements, as apportioned earlier via PCA. It is also the predominant occupation of the settlers and due to water availability challenges during the dry season, they depend largely on rainfall and availability of more water during the wet season. Hence, the higher concentrations of the metals during the wet season were very likely because, within the farm settlements, agricultural activities increased during the wet season.
For microbial populations, 2 components were extracted via PCA for the Ajegunle farm settlement. The PC 1 was a result of fecal contamination59 through livestock and human wastes. Faecal pollution is a major contaminant in both surface and groundwater resources in Nigeria59,62. The PC 2 was indicated to have resulted from the use of manures and livestock agricultural wastes55. For the Akufo farm settlement, the only principal component was probably a result of livestock agricultural wastes, sewage effluents and organic decomposition. From the Eruwa farm settlement, however, 2 PCs were extracted. The PC 1 was mostly due to livestock agricultural wastes. The negative loading of the coliform count indicated that it was not necessarily dependent on the others with positive loading59. Escherichia coli count had strong positive loading on PC 2 indicating faecal contamination59.
The spatial variation maps generated for the heavy and trace metals, as well as for the microbial populations showed the seasonal distributions of the metals and microbial populations within the farm settlements. The maps show the quality zones within the settlements during the dry and wet seasons, thereby providing a guide for identifying places with the best quality/suitability of the water for different purposes, such as fish farming, piggery, vegetable farming, etc.63,64. They are useful for locating less polluted areas for wells or borehole drilling. Agricultural activities can then be systematically positioned according to water needs and safety. The GIS-based IDW maps are capable of enhancing sustainable management of water resources within the farm settlements65, they are excellent tools for summarizing overall water quality conditions over space and time37. Similar variation maps have been generated by Sahoo et al.12, Gidey13 and Zaharaddeen66 among others.
Effective and sustainable agricultural (especially livestock) waste management strategies as well as pollution control against agricultural run-off and chemicals are necessary within the farm settlements to curb contamination and ensure good health for the settlers.
The water samples from the three farm settlements showed possibilities of cadmium, iron, manganese E. coli and streptococcal contaminations. Bedrock weathering, fertilizer/agricultural waste/run-off leachates, agricultural activities, fecal contamination, livestock agricultural wastes, sewage effluents and organic decomposition were identified as pollution sources for the metal and microbial contaminations in the settlements. The spatial maps revealed the existing distributions of selected metals and microbial populations within Ajegunle and Akufo farm settlements, which would enhance the systematic utilization of the water for agriculture.
It is hereby recommended that routine groundwater quality assessment be carried out to follow the dynamics and be able to manage and control pollution within the farm settlements. Also, appropriate treatments should be administered to the groundwater, especially, to take care of magnesium, iron, cadmium, manganese, E. coli and Streptococcal contaminations. Since, this study identified high magnesium concentrations in the study areas, magnesium-tolerant crops will thrive better. Finally, effective and sustainable agricultural (especially livestock) waste management strategies as well as pollution control against agricultural run-off and chemicals are necessary within the farm settlements to curb contamination and ensure good health for the settlers.
A limitation of this study is that the water sources within the Eruwa farm settlement were very few and wide apart and hence spatial maps could not be generated for the settlement.
CONCLUSION
The water samples from the three farm settlements showed possibilities of cadmium, iron, manganese Escherichia coli and streptococcal contaminations and therefore should be monitored and treated accordingly before use for agricultural or other purposes. Also, crops that are resistant to, or require high concentrations of cadmium, iron and manganese are recommended for cultivation within the farm settlements. The information from this research will help safeguard the lives of the farm settlers as well as the consumers of agricultural produce from the settlements against metal and microbial contamination.
SIGNIFICANCE STATEMENT
This study assessed the contamination, apportioned pollution sources using principal component analysis (PCA) and mapped the spatial variabilities of heavy and trace metals as well as microbial populations in the water available for agriculture at the Ajegunle, Akufo and Eruwa farm settlements in southwestern Nigeria. The information from this research will help safeguard the lives of the farm settlers as well as the consumers of agricultural produce from the settlements against metal and microbial contamination. The water samples from the three farm settlements showed possibilities of cadmium, iron, manganese, Escherichia coli and streptococcal contaminations and therefore should be monitored and treated accordingly before use for agricultural or other purposes.
ACKNOWLEDGMENTS
The authors wish to acknowledge Mr. Oladapo of the Institute of Agricultural Research and Training (I.A.R.&T. Ibadan) and Mr. Timilehin Alakoya for their contributions to the success of the study in the aspects of laboratory and statistical analyses, respectively. Our gratitude also goes to the Land and Water Resources Management and the Agricultural and Environmental Engineering Units of I.A.R.&T. for hosting Folarin G.M. during the internship periods.
REFERENCES
- Oluwatosin, G.A., O.D. Adeoyolanu, A.O. Ojo, K.S. Are, T.O. Dauda and V.O. Aduramigba-Modupe, 2009. Heavy metal uptake and accumulation by edible leafy vegetable (Amaranthus Hybridus L.) grown on urban valley bottom soils in Southwestern Nigeria. Soil Sediment Contam. Int. J., 19: 1-20.
- Beauchat, L.R. and J.H. Ryu, 1997. Produce handling and processing practices. Emerging Infect. Dis., 3: 459-465.
- Rohr, J.R., C.B. Barrett, D.J. Civitello, M.E. Craft and B. Delius et al., 2019. Emerging human infectious diseases and the links to global food production. Nat. Sustainability, 2: 445-456.
- Suruchi and P. Khanna, 2011. Assessment of heavy metal contamination in different vegetables grown in and around urban areas. Res. J. Environ. Toxicol., 5: 162-179.
- Ayangbenro, A.S. and O.O. Babalola, 2017. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health, 14.
- Jalali, M., 2010. Application of multivariate analysis to study water chemistry of groundwater in a semi-arid aquifer, Malayer, Western Iran. Desalin. Water Treat., 19: 307-317.
- Akbal, F., L. Gürel, T. Bahadır, İ. Güler, G. Bakan and H. Büyükgüngör, 2011. Water and sediment quality assessment in the mid-Black Sea coast of Turkey using multivariate statistical techniques. Environ. Earth Sci., 64: 1387-1395.
- Kura, N.U., M.F. Ramli, W.N.A. Sulaiman, S. Ibrahim, A.Z. Aris and A. Mustapha, 2013. Evaluation of factors influencing the groundwater chemistry in a small tropical island of Malaysia. Int. J. Environ. Res. Public Health, 10: 1861-1881.
- Folarin, G.M., B.S. Badmus, O.D. Akinyemi, O.A. Idowu, A.O. Oke and G.O. Badmus, 2023. Groundwater quality assessment using physico-chemical parameters and pollution sources apportionment in selected farm settlements of Southwestern Nigeria. Int. J. Energy Water Res. 7: 85-103.
- Oyinkuro, O.A. and E.D. Rowland, 2017. Spatial groundwater quality assessment by WQI and GIS in Ogbia LGA of Bayelsa State, Nigeria. Asian J. Phys. Chem. Sci., 4.
- Naoum, S. and I.K. Tsanis, 2004. Ranking spatial interpolation techniques using a GIS-based DSS. Global Nest: J., 6: 1-20.
- Sahoo, S., A. Kaur, P. Litoria and B. Pateriya, 2014. Geospatial modelling for groundwater quality mapping: A case study of Rupnagar District, Punjab, India. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci., XL-8: 227-232.
- Gidey, A., 2018. Geospatial distribution modeling and determining suitability of groundwater quality for irrigation purpose using geospatial methods and water quality index (WQI) in Northern Ethiopia. Appl. Water Sci., 8.
- Oke, A., A. Sangodoyin, K. Ogedengbe and T. Omodele, 2013. Mapping of river waterquality using inverse distance weighted interpolation in Ogun-Osun River Basin, Nigeria. Acta Geogr. Debrecina Landscape Environ. Ser., 7: 48-62.
- Oke, M.O., O. Martins, O.A. Idowu and O. Aiyelokun, 2015. Comparative analysis of groundwater recharge estimation value obtained using empirical methods in Ogun and Oshun River Basins. Ife J. Sci., 17: 53-63.
- Ogunsanwo, F.O., J.A. Olowofela, I.C. Okeyode, O.A. Idowu and O.T. Olurin, 2019. Aeroradiospectrometry in the spatial formation characterization of Ogun State, South-Western, Nigeria. Sci. Afr., 6.
- APHA-AWWA-WPCF, 1998. Standard Methods for the Examination of Water and Wastewater. 20th Edn., American Public Health Association, Washington, DC, ISBN: 9780875532356 .
- Assubaie, F.N., 2015. Assessment of the levels of some heavy metals in water in Alahsa Oasis farms, Saudi Arabia, with analysis by atomic absorption spectrophotometry. Arabian J. Chem., 8: 240-245.
- Ahmed, T., S. Baidya, M. Acharjee and T. Rahman, 2013. Qualitative analysis of drinking water through the most probable number (MPN) method. Stamford J. Microbiol., 3: 9-16.
- Tsade, H.K., 2016. Atomic absorption spectroscopic determination of heavy metal concentrations in Kulufo River, Arbaminch, Gamo Gofa, Ethiopia. J. Environ. Anal. Chem., 3.
- Radulescu, C., I.D. Dulama, C. Stihi, I. Ionita, A. Chilian, C. Necula and E.D. Chelarescu, 2014. Determination of heavy metal levels in water and therapeutic mud by atomic absorption spectrometry. Rom. J. Phys., 59: 1057-1066.
- WHO, 2011. Guidelines for Drinking-Water Quality. 4th Edn., World Health Organization, Geneva, Switzerland, ISBN: 9789241548151, Pages: 564.
- Oloruntoba, E.O. and T.O. Ogunbunmi, 2020. Impact of informal auto-mobile mechanic workshops activities on groundwater quality in Ibadan, Nigeria. J. Water Resour. Prot., 12: 590-606.
- Kaiser, H.F., 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23: 187-200.
- Kaiser, H.F. and J. Rice, 1974. Little Jiffy, mark IV. Educ. Psychol. Meas., 34: 111-117.
- Liu, C.W., K.H. Lin and Y.M. Kuo, 2003. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ., 313: 77-89.
- Mohapatra, P.K., R. Vijay, P.R. Pujari, S.K. Sundaray and B.P. Mohanty, 2011. Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri City, India: A multivariate statistical approach. Water Sci. Technol., 64: 809-817
- Mustapha, A. and A.Z. Aris, 2012. Multivariate statistical analysis and environmental modeling of heavy metals pollution by industries. Pol. J. Environ. Stud., 21: 1359-1367.
- Khouni, I., G. Louhichi and A. Ghrabi, 2021. Use of GIS based inverse distance weighted interpolation to assess surface water quality: Case of Wadi El Bey, Tunisia. Environ. Technol. Innovation., 24.
- Pande, C.B. and K. Moharir, 2018. Spatial analysis of groundwater quality mapping in hard rock area in the Akola and Buldhana Districts of Maharashtra, India. Appl. Water Sci., 8.
- Adepoju-Bello, A.A. O.O. Ojomolade, G.A. Ayoola and H.A.B. Coker, 2009. Quantitative analysis of some toxic metals in domestic water obtained from Lagos metropolis. Niger. J. Pharm., 42: 57-60.
- Marcovecchio, J.E., J.E. Botte and R.H. Freije, 2007. Heavy Metals, Major Metals, Trace Elements. In: Handbook of Water Analysis, Nollet, L.M.L. and L.S.P. de Gelder (Eds.), CRC Press, Boca Raton, Florida, ISBN: 9780429123597, pp: 275-311.
- Adepelumi, A., B. Ako and T. Ajayi, 2001. Groundwater contamination in the basement-complex area of Ile-Ife, Southwestern Nigeria: A case study using the electrical-resistivity geophysical method. Hydrogeol. J., 9: 611-622.
- Tewodros, B.A., T.W. Behailu and T.S. Badessa, 2017. Analysis of physical and chemical parameters in ground water used for drinking around Konso Area, Southwestern Ethiopia. J. Anal. Bioanal. Tech., 8.
- Kayode, A.A.A., J.O. Babayemi, E.O. Abam and O.T. Kayode, 2011. Occurrence and health implications of high concentrations of Cadmium and Arsenic in drinking water sources in selected towns of Ogun State, South West, Nigeria. J. Toxicol. Environ. Health Sci., 3: 385-391.
- Zhao, H., J. Guan, Q. Liang, X. Zhang, H. Hu and J. Zhang, 2021. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep., 11.
- Al Maliki, A.A., Z.D. Abbass, H.M. Hussain and N. Al-Ansari, 2020. Assessment of the groundwater suitability for irrigation near Al Kufa City and preparing the final water quality maps using spatial distribution tools. Environ. Earth Sci., 79.
- Peana, M., A. Pelucelli, C.T. Chasapis, S.P. Perlepes, V. Bekiari, S. Medici and M.A. Zoroddu, 2023. Biological effects of human exposure to environmental cadmium. Biomolecules, 13.
- Bouchard, M., F. Laforest, L. Vandelac, D. Bellinger and D. Mergler, 2007. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect., 115: 122-127.
- Anyanwu, E.D. and O.G. Onyele, 2018. Occurrence and concentration of heavy metals in a rural spring in South-Eastern Nigeria. J. Appl. Sci. Environ. Manage., 22: 1473-1478.
- Rai, P.K., S.S. Lee, M. Zhang, Y.F. Tsang and K.H. Kim, 2019. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int., 125: 365-385.
- Afolabi, T.A., C.C. Ogbuneke, O.A. Ogunkunle and F.O. Bamiro, 2012. Comparative assessment of the potable quality of water from industrial, urban and rural parts of Lagos, Nigeria. Ife J. Sci., 14: 221-232.
- Zheng, W., M. Aschner and J.F. Ghersi-Egea, 2003. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol., 192: 1-11.
- de Freitas, J.M. and R. Meneghini, 2001. Iron and its sensitive balance in the cell. Mutat. Res. Fundam. Mol. Mech. Mutagen., 475: 153-159.
- Emenike, P.C., C.C. Nnaji and I.T. Tenebe, 2018. Assessment of geospatial and hydrochemical interactions of groundwater quality, Southwestern Nigeria. Environ. Monit. Assess., 190.
- Bitton, G., S.R. Farrah, R.H. Ruskin, J. Butner and Y.J. Chou, 1983. Survival of pathogenic and indicator organisms in ground water. Ground Water, 21: 405-410.
- Schijven, J.F. and S.M. Hassanizadeh, 2000. Removal of viruses by soil passage: Overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol., 30: 49-127.
- Pang, L., M. Close, M. Goltz, L. Sinton, H. Davies, C. Hall and G. Santon, 2004. Estimation of septic tank setback distances based on transport of E. coli and F-RNA phages. Environ. Int., 29: 907-921.
- Pandey, P.K., P.H. Kass, M.L. Soupir, S. Biswas and V.P. Singh, 2014. Contamination of water resources by pathogenic bacteria. AMB Express, 4.
- Adekunle, I.M., M.T. Adetunji, A.M. Gbadebo and O.B. Banjoko, 2007. Assessment of groundwater quality in a typical rural settlement in Southwest Nigeria. Int. J. Environ. Public Health, 4: 307-318.
- Figueras, M.J. and J.J. Borrego, 2010. New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health, 7: 4179-4202.
- Jang, J., H.G. Hur, M.J. Sadowsky, M.N. Byappanahalli, T. Yan and S. Ishii, 2017. Environmental Escherichia coli: Ecology and public health implications-A review. J. Appl. Microbiol., 123: 570-581.
- Nwidu, L.L., B. Oveh, T. Okoriye and N.A. Vaikosen, 2008. Assessment of the water quality and prevalence of water borne diseases in Amassoma, Niger Delta, Nigeria. Afri. J. Biotechnol., 7: 2993-2997.
- Terzieva, S.I. and G.A. McFeters, 2011. Survival and injury of Escherichia coli, Campylobacter jejuni, and Yersinia enterocolitica in stream water. Can. J. Microbiol., 37: 785-790.
- Ishaku, J.M., U. Kaigama and N.R. Onyeka, 2011. Assessment of groundwater quality using factor analysis in Mararaba-Mubi Area, Northeastern Nigeria. J. Earth Sci. Geotech. Eng., 1: 9-33.
- Kwami, I.A., J.M. Ishaku, Y.S. Hamza, A.M. Bello and S. Mukkafa, 2019. Application of multivariate statistical techniques for the interpretation of groundwater quality in gombe and environs, North-East Nigeria. J. Geosci. Geomatics, 7: 9-14.
- Elumalai, V., K. Brindha and E. Lakshmanan, 2017. Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: A case study from South Africa. Water, 9.
- Shrestha, S., D.J. Semkuyu and V.P. Pandey, 2016. Assessment of groundwater vulnerability and risk to pollution in Kathmandu Valley, Nepal. Sci. Total Environ., 556: 23-35.
- Taiwo, A.M., 2012. Source identification and apportionment of pollution sources to groundwater quality in major cities in Southwest, Nigeria. Geofizika, 29: 157-174.
- Idoko, O.M., T.E. Ologunorisa and A.A. Okoya, 2012. Temporal variability of heavy metals concentration in rural groundwater of Benue State, Middle Belt, Nigeria. J. Sustainable Dev., 5: 2-16.
- Eseyin, O. Olorunfemi., G.J. Udom and C.I. Osu, 2018. Concentrations and seasonal variation in heavy metal contamination of leachates, borehole water, soil and edible plants near unengineered dumpsites in Port Harcourt, Nigeria. Int. J. Sci. Res. Publ., 8: 492-505.
- Ochieng, G.M., O.I. Ojo, K. Ogedengbe and J.M. Ndambuki, 2011. Open wells, sanitary features, pollutions and water qualities: Case study of Ibadan slums, Nigeria. Int. J. Phys. Sci., 6: 3062-3073.
- Ketata-Rokbani, M., M. Gueddari and R. Bouhlila, 2011. Use of geographical information system and water quality index to assess groundwater quality in el khairat deep aquifer (Enfidha, Tunisian Sahel). Iran. J. Energy Environ., 2: 133-144.
- Khadri, S.F.R, C. Pande and K. Moharir, 2013. Groundwater quality mapping of PTU-1 watershed in Akola District of Maharashtra India using geographic information system techniques. Int. J. Sci. Eng. Res., 4: 1856-1869.
- Tiwari, G. and J.P. Shukla, 2015. A review on remote sensing and GIS techniques in water resource development and management with special reference to groundwater. Int. J. Remote Sens. Geosci., 4: 10-16.
- Zaharaddeen, I., 2015. Assessment of spatial and temporal distribution of some physiochemical parameters of groundwater quality of Birnin Gwari, North West of Nigeria. Int. J. Technol. Enhancements Emerging Eng. Res., 3: 43-52.
How to Cite this paper?
APA-7 Style
Folarin,
G.M., Badmus,
B.S., Akinyemi,
O.D., Idowu,
O.A., Oke,
A.O., Omodele,
T., Badmus,
G.O. (2023). Metal and Microbial Contamination in the Water Available for Agriculture in Selected Farm Settlements in Nigeria. Trends in Agricultural Sciences, 2(3), 348-368. https://doi.org/10.17311/tas.2023.348.368
ACS Style
Folarin,
G.M.; Badmus,
B.S.; Akinyemi,
O.D.; Idowu,
O.A.; Oke,
A.O.; Omodele,
T.; Badmus,
G.O. Metal and Microbial Contamination in the Water Available for Agriculture in Selected Farm Settlements in Nigeria. Trends Agric. Sci 2023, 2, 348-368. https://doi.org/10.17311/tas.2023.348.368
AMA Style
Folarin
GM, Badmus
BS, Akinyemi
OD, Idowu
OA, Oke
AO, Omodele
T, Badmus
GO. Metal and Microbial Contamination in the Water Available for Agriculture in Selected Farm Settlements in Nigeria. Trends in Agricultural Sciences. 2023; 2(3): 348-368. https://doi.org/10.17311/tas.2023.348.368
Chicago/Turabian Style
Folarin, Gbolahan, Muyiwa, Biodun Suraj Badmus, Olukayode Dewunmi Akinyemi, Olufemi Abiola Idowu, Adebayo Olubukola Oke, Taiwo Omodele, and Ganiyu Olabode Badmus.
2023. "Metal and Microbial Contamination in the Water Available for Agriculture in Selected Farm Settlements in Nigeria" Trends in Agricultural Sciences 2, no. 3: 348-368. https://doi.org/10.17311/tas.2023.348.368

This work is licensed under a Creative Commons Attribution 4.0 International License.



