Soil Acidity Ameliorative Potentials of Biochar from Sawdust and Tithonia diversifolia Feedstock
| Received 15 Jan, 2023 |
Accepted 12 Jun, 2023 |
Published 30 Sep, 2023 |
Background and Objective: Aluminum toxicity and phosphorus deficiency are adverse conditions in tropical acid soils which conventional liming materials (CLM) alone cannot ameliorate sustainably. Biochar from sawdust (SD) and Tithonia diversifolia (TD) biomass with or without poultry manure (PM) were evaluated for their liming and fertilizer potentials. Materials and Methods: Four biochars: SD+PM, SD-PM, TD+PM and TD-PM prepared at 350 and 450°C pyrolysis temperatures were applied at 0.38 g/150 g soil. Soil treated with CLM (CaCO3) at 0.08 g/150 g soil and absolute control (AC) were compared. Amended soils were moistened to field capacity and incubated for four weeks, sown to cowpea and nurtured for two weeks. Results: The biochars had comparatively high liming efficiencies (75-106%) as the CaCO3 (100%) but outperformed CaCO3 in macronutrient enrichment, carbon sequestration and cowpea root enhancement. The CaCO3 however was higher (4.88 cmol kg‾1) in calcium enrichment compared to the biochars (3.67-4.62 cmol kg‾1). This did not confer higher exchangeable acidity (1.42 cmol kg‾1) detoxification potential on CaCO3 from the initial 2.20 cmol kg‾1 compared to the biochars (1.20-1.48 cmol kg‾1). Conclusion: Increasing exchangeable Ca, K and available P, therefore, are major immediate pathways for acidity amelioration in biochar-amended acid soils as indicated by their negative correlations with exchangeable acidity.
| Copyright © 2023 Oyeyiola and Ogunlaran. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Acid soils are soils with a pH of less than 7. The acidity state of soil becomes a matter of concern when the pH drops below 5.5 when the solubility of toxic aluminum and macronutrient deficiencies becomes evident1,2. About 40% (equivalent to 1.5 g ha–1) of world soils are acidic while about 22% (equivalent to 659 M ha) of farmland in Africa is acidic3. Approximately one-third of tropical soil was reported to be strongly acidic4. Acid soils are widely spread in Southeastern and a portion of Southwestern Nigeria5,6. These agroecological zones are characterized by high annual rainfall distribution of above 1200 mm and could reach 3500 mm in the Southeastern part of Nigeria. The high rainfall intensities encourage the leaching of soluble basic nutrients (Ca2+, Mg2+ and K+) and replacement by Al3+ and H+ The soils in these zones developed from acidic and alluvial parent material such as sandstone, shales, coastal plain sand, basement complex rocks, volcanic ash, alluvial sediments and granite5,7. The increasing farmland areas lost to soil acidification in these agro-ecological zones are further aggravated by indiscriminate usage of chemical fertilizers dominantly urea and NPK fertilizer grades among the farmers8-10.
Acid soils are characterized by low base saturation and high acidity saturation as a result of low concentration of basic cations (Ca2+, Mg2+, K+ and Na+) and high concentration of acidic cations (Al3+, H+, Fe2+ and Mn2+)1,11. This group of problematic soil is peculiar with the Ferralsols, Nitisols, Acisols and Plinthisols1. Their major problems are micronutrient toxicity (including Al, Fe and Mn toxicities) and macronutrient deficiencies (especially P deficiency)1,10. These problems culminate in poor rooting systems in plants leading to poor nutrient uptake, growth and yield performances of plants12-14. Soil acidity has been managed conventionally through the use of common liming materials such as calcitic limestone, quick lime and slaked lime. The sole use of these conventional liming materials has been bedeviled by their relative scarcity, high cost of procurement, caustic nature that predisposes their users to a respiratory disorder and contribution to organic carbon depletion in soils15-18. These limitations have led to the search for more sustainable approaches to mitigating soil acidity in the world which has placed organic amendments rich in ash alkalinity in the limelight. Organic materials such as animal manure, compost, phospho-compost and biochar have been reported to neutralize soil acidity through multiple approaches19. Such include their ability to improve soil structure, aeration, microbial activity, water and nutrient retention capacities. Common acidity amelioration mechanisms by these organic materials are their capacities to increase soil pH through the mineralization of their innate ash alkalinity, enhancement of soil P pool and soil organic carbon sequestration for chelating the toxic Al3+ 20,21.
Biochar is a solid carbon-rich product from the thermal decomposition of biomass in the absence of oxygen. Its production and use have attracted immense attention from many countries of the world because of its widely acclaimed potential for sustainable soil and environmental management in the face of current climate change effects ravaging the world. Many research works have been channeled into the use of biochar in the revitalization of problematic soils in the world but little of such has been documented for acid soils in Nigeria. Earlier studies have focused much on the production and use of biochar derived from woody biomass and agro-wastes such as sawdust and crop residues. Little attention has been given to the use of biomass from weeds and co-pyrolysis of plant biomass with animal manure for biochar production. Commonly distributed annual luxuriant weeds such as Tithonia diversifolia and Imperata cylindrica in Southwestern Nigeria can be a potential feedstock for biochar production against woody products with existing and increasing competitive uses. Biochar produced at a slow pyrolysis temperature range of 300-450°C are reported for their potentially higher fertilizer properties as macronutrients such as N and P are lost as pyrolysis temperature increases22. The peculiarity of the acid soil studied characterized by low macronutrients (N, P and basic cations) and organic carbon calls for the need for its management with specially modified amendments that would serve as both liming and fertilizer materials. Therefore, this work explored biochar production from woody and non-woody plant biomass with or without poultry manure at slow pyrolysis temperatures of 350 and 450°C. The acidity neutralizing, macronutrient enrichment and carbon sequestering potentials of biochar produced were comparatively studied in degraded acid soil from Southwestern Nigeria.
MATERIALS AND METHODS
Soil sampling and routine analysis: The trial was conducted between February 3rd and April 29th, 2022. The acid soil studied was obtained from the Arable Farm of Leventis School of Agriculture, Imo, Ilesha, Osun State, Nigeria. The soil originates from amphibole, biotite schist and gneiss-rich minerals under tropical rainforest agro-ecological conditions23. The soil was classified as a Ultisols following23 by Amusan and Ashaye24 and as Itagunmodi soil series using local classification by Smyth and Montgomery7. Topsoil (0-15 cm depth) sampled from the field was air-dried, crushed, pulverized, sieved and analyzed routinely.
The soil pH was determined by shaking 10 g of the soil in distilled water in a ratio of 1:2 on a mechanical shaker for 30 min followed by reading the pH of the solution using a glass electrode calibrated in buffer solutions 4, 7 and 9. Organic carbon was determined using the dichromate wet oxidation method described by Walkley and Black25. Available phosphorus in the soil was extracted by Bray-1 solution while total N was determined using the Macro-Kjeldahl method as described in IITA26. Exchangeable cation (Ca, Mg, K, Na) and acidity were extracted by neutral 1N NH4OAC and 1N KCl, respectively. The hydrometer method as described in IITA26 was followed for the determination of the particle size distribution of the experimental soil.
Biochar preparation: The Biochars tested were produced from two feedstocks, Tithonia diversifolia (TD) and sawdust (SD) from Gmelina arboreae with or without poultry manure (PM) at two pyrolysis temperatures of 350 and 450°C. The feedstocks were pre-treated by oven-drying at 105°C for 24 hrs to remove volatile gases for optimization of the pyrolysis processes done in the muffled furnace at 20 min residence time. As 30 g of TD or SD was used for the production of sole TD (TD-PM) and SD (SD-PM) biochars, respectively while 15 g of TD or SD mixed with 15 g of PM were used for the production of TD+PM and SD+PM biochars, respectively. The resultant biochars were thereafter cooled, crushed to fine particle sizes and sealed up in nylon bags. Triplicate samples of each biochar were subjected to nutrient analysis following procedures largely meant for plant analysis as documented by Oyeyiola et al.27.
Treatments and experimental design: The incubation trial was carried out between April and September, 2021 at the laboratory of the Department of Crop Production and Soil Science, Ladoke Akintola University of Technology Ogbomoso, Oyo State, Nigeria. The experiment was a factorial combination of four biochar types (from different feedstock combinations viz: SD-PM, TD-PM, SD+PM and TD+PM) and two pyrolysis temperatures of 350 and 450°C. Soil treated with a conventional liming material (CaCO3) and an absolute control soil that received neither biochar nor CaCO3 were included for comparison. Each incubation cup measuring 8 cm in diameter and 4.4 cm high was filled with 150 g soil. Appropriate biochar treatments were applied at 5 t ha–1 (equivalent to 0.38 g/150 g soil). The CaCO3 was applied at 1 t ha–1 (equivalent to 0.08 g/150 g soil). The applied biochar and liming material were homogenized into the soil and moistened to field capacity using 40 mL of distilled water. The whole set-up was kept covered with foil paper to minimize water loss and prevent contamination and was incubated in the dark at a temperature that fluctuated between 25 and 27°C. There were four cycles of wetting and drying during the incubation trial that lasted for four weeks. Thereafter, cowpea seeds earlier surface sterilized with 75% ethanol were sown into each incubated soil at one plant per incubation cup and were nurtured for two weeks.
Data collection: Soil pH was monitored at 24 hrs, 7 days and 4 weeks of incubation and at cowpea seedling harvesting. The soil pH values determined at harvesting were used for the estimation of liming efficiencies of the different biochar types using a formula adopted by An-Ning et al.18 and Anetor and Akinrinde28:
Soils sampled at cowpea seedling harvesting were analyzed for available phosphorus, organic carbon, exchangeable bases and acidity following the procedures earlier described by Juo26. Data on root length and dry biomass weight of the cowpea seedlings were also taken at harvesting.
Statistical analysis: All the data collected were subjected to a two-way analysis of variance using the Genstat statistical package (8th edition) and significant means were separated by LSD at a 5% probability level. Correlation analysis was carried out to ascertain the relationship among different soil and plant parameters following biochar application in the acid soil.
RESULTS AND DISCUSSION
Characteristics of the experimental soil and biochar tested: The studied soil was strongly acidic as indicated by its low pH (4.35), basic cation (Ca, Mg, K and Na) contents and high exchangeable acidity (2.20 cmol kg–1) (Table 1). This gave base and acidity saturation of 46 and 54%, respectively. The soil was additionally deficient in available P and organic carbon.
| Table 1: | Characteristics of the acid soil studied | |||
| Parameters | Values |
| Soil pH (H2O) | 4.35 |
| Available P (Bray-P mg kg–1) | 3.86 |
| Organic Carbon (%) | 1.38 |
| Total N (g kg–1) | 1.3 |
| Ex. Cations (cmol kg–1) | |
| Ca | 0.96 |
| Mg | 0.51 |
| K | 0.23 |
| Na | 0.18 |
| H | 0.96 |
| Al | 1.24 |
| ECEC | 4.08 |
| Base saturation (%) | 46 |
| Acidity saturation (%) | 54 |
| Sand (g kg–1) | 520 |
| Silt (g kg–1) | 150 |
| Clay (g kg–1) | 330 |
| Textural class | Sandy clay loam |
| ECEC: Effective cation exchange capacity | |
| Table 2: | pH and nutrient characteristics of the biochar tested in the acid soils | |||
Sawdust based biochar ----------------------------------------------------- |
T. diversifolia based biochar -------------------------------------------------- |
|||||||
| Biochar parameter | SD+PM ----------------------- |
SD-PM ---------------------- |
TD+PM ---------------------- |
TD-PM --------------------- |
||||
| 350°C | 450°C | 350°C | 450°C | 350°C | 450°C | 350°C | 450°C | |
| pH | 10.2 | 11.1 | 9.15 | 9.7 | 10.91 | 11.5 | 9.8 | 10.75 |
| Ash (%) | 8.51 | 53.12 | 5.6 | 8.82 | 57.31 | 68.61 | 10.23 | 10.14 |
| Ca (%) | 2.73 | 6.52 | 1.36 | 1.59 | 6.43 | 8.15 | 1.48 | 1.45 |
| Mg (%) | 0.35 | 0.91 | 0.22 | 0.45 | 1.07 | 1.41 | 0.49 | 0.52 |
| K (%) | 0.97 | 1.41 | 0.75 | 0.98 | 1.85 | 1.71 | 2.56 | 2.5 |
| Organic C (%) | 51.5 | 29.45 | 54.11 | 50.02 | 26.35 | 19.87 | 50.44 | 49.23 |
| N (%) | 0.35 | 0.68 | 0.31 | 0.42 | 0.85 | 0.91 | 0.81 | 0.9 |
| P (%) | 0.13 | 1.27 | 0.07 | 0.16 | 1.52 | 1.41 | 0.31 | 0.29 |
| SD+PM@350, SD+PM@450, TD+PM@350 and TD+PM@450 are sawdust and tithonia biochar co-pyrolyzed with poultry manure at 350 and 450°C, respectively, while, SD-PM@350, SD-PM@450, TD-PM@350 and TD-PM@450 are sole sawdust and tithonia biochar pyrolyzed at 350 and 450°C, respectively | ||||||||
All the biochar tested were alkaline with a pH range of 9.15 (SD-PM) to 11.50 (TD+PM) making them potentially suitable liming materials as illustrated in Table 2. Biochar co-pyrolyzed with poultry manure (PM) and produced at higher (450°C) pyrolysis temperature had higher pH, ash, N and P contents. This thus conferred higher liming and fertilizer properties on this group of biochar. Conversely, biochar produced without PM at a lower pyrolysis temperature of 350°C was higher in organic C content but lower in pH, ash, N and P. This may be indicating this latter group of biochar better suitable for higher carbon sequestration in soils than the former group.
pH changes in an acid soil following the application of biochar from different feedstock combinations and pyrolysis temperatures: Soil pH during the incubation and cropping periods was consistently affected by the biochar feedstock combination (Table 3). Biochar co-pyrolyzed with PM was responsible for significantly higher soil pH in the acid soil at two and four weeks of incubation (WOI) and after cowpea seedling harvesting. The potentials of PM spiked biochar to improve soil pH at cowpea seedling harvesting varied with basal biomass type and pyrolysis temperature. The efficacy of all the biochar tested to improve soil pH appreciated with increasing incubation period and was highest after cowpea seedling harvesting. This is indicating the incremental rate of basic cation mineralization from all the biochar tested as incubation time progresses. The soil pH increased from an initial 4.35 to a range of 5.12 (TD-PM at 350°C) to 5.14 (TD+PM at 450°C) at 4 WOI and to 5.56 (TD-PM at 350°C) to 5.95 (SD+PM at 450°C) at cowpea seedling harvesting.
| Table 3: | pH changes in an acid soil following the application of biochar from different feedstock combinations and pyrolysis temperatures | |||
| Biochar feedstock | Soil pH @ 2WOI ------------------------------- |
Soil pH @ 4WOI ------------------------------- |
Soil pH @ harvesting ------------------------------- |
||||||
Pyrolysis temperature ------------------- |
Pyrolysis temperature ------------------- |
Pyrolysis temperature ------------------- |
|||||||
350°C |
450°C |
Mean |
350°C |
450°C |
Mean |
350°C |
450°C |
Mean |
|
| SD+PM | 5.1 |
5.21 |
5.16 |
5.22 |
5.27 |
5.24 |
5.92 |
5.95 |
5.94 |
| SD-PM | 5.18 |
5.07 |
5.13 |
5.21 |
5.09 |
5.15 |
5.71 |
5.74 |
5.73 |
| TD+PM | 5.24 |
5.22 |
5.23 |
5.47 |
5.35 |
5.41 |
5.6 |
5.87 |
5.73 |
| TD-PM | 5.08 |
5.04 |
5.06 |
5.12 |
5.14 |
5.13 |
5.56 |
5.87 |
5.71 |
| Mean | 5.15 |
5.14 |
5.25 |
5.21 |
5.7 |
5.86 |
|||
| Biochar feedstock (LSD) | 0.069** |
0.105*** |
0.163* |
||||||
| Pyrolysis temperature (LSD) | ns |
ns |
0.115** |
||||||
| BF×PT (LSD) | 0.097* |
ns |
0.23ns |
||||||
| Checks | |||||||||
| CaCO3 | 5.49 |
5.88 |
5.88 |
||||||
| AC | 4.77 |
4.68 |
4.62 |
||||||
| SD+PM@350, SD+PM@450, TD+PM@350 and TD+PM@450 are sawdust and tithonia biochar co-pyrolyzed with poultry manure at 350 and 450°C respectively while SD-PM@350, SD-PM@450, TD-PM@350 and TD-PM@450 are sole sawdust and tithonia biochar pyrolyzed at 350 and 450°C, respectively, AC: Absolute control that received neither biochar nor CaCO3, WOI: Weeks of incubation, *,**,***Significant at p<0.05, 0.01 and 0.001, respectively and ns: Not significant | |||||||||
The potential of the conventional lime (CaCO3) to improve soil pH was superior to those of the biochars at 2 and 4 WOI. The pH of soils amended with CaCO3 however, remained unchanged after cowpea seedling harvesting while those amended with biochars increased significantly with higher pH of 5.95 obtained from soil amended with SD+PM at 450°C biochar over 5.88 from soil amended with CaCO3. This is indicating improved biochar liming and basic nutrient mineralization efficiencies with aging in the soil. These positive residual tendencies will reduce the annual cost implications of purchasing fertilizers and amendments for field application. Similar positive biochar aging effects on soil pH have been documented29.
Liming efficiencies of the biochar tested as influenced by feedstock types and pyrolysis temperatures: All the biochar tested had high (75-106%) liming efficiencies (LE) with two of the biochars: SD+PM@350 and SD+PM@450 having LE values higher than the 100% referenced for the conventional lime (CaCO3) as shown in Fig. 1. Biochar produced from SD had higher liming potentials over corresponding TD biochar despite higher pH, ash and basic cations contents in TD based biochar over SD based biochar (Fig. 1). The LE ranged from 75 (TD-PM@350) to 106 (SD+PM@450) relative to 100% from the conventional CaCO3. The perennial nature of the tree species from where the sawdust used for the trail was obtained had encouraged the accumulation of absorbed basic cations in the tree plant tissues over the years. There is, however, relatively lower absorbed basic cations by the annual weed T. diversifolia studied. Furthermore, large concentrations of the basic nutrients quickly mineralized into the soil by the TD-based biochar seemed to be easily absorbed and utilized by the growing plants rather than left in the soil to buffer soil pH via cation exchange as may be observed in SD-based biochar. Biochar co-pyrolyzed with PM regardless of the basal feedstock had higher LE over similar biochar without PM. Earlier studies had demonstrated PM to have high ash alkalinity properties over other biochar feedstock30. The ash alkalinity of soil amendment is an important chemical characteristic that dominantly influences liming potentials of any amendment31. In this study, the inclusion of PM in biochar increased LE by 14.9% compared to biochar without PM. Increasing pyrolysis temperature supported increases in LE of the resultant biochar with greater responses from TD biochar. The LE, therefore, increased by 2.5, 3.1, 33.0 and 26.4% in SD+PM, SD-PM, TD+PM and TD-PM, respectively as pyrolysis temperature increased from 350 to 450°C. Similar observations were documented in these findings32,33.
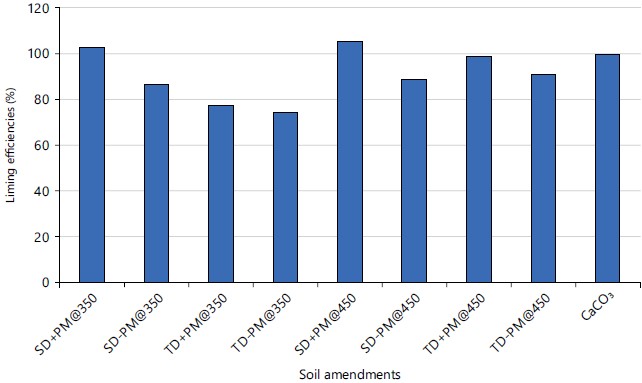
|
| Table 4: | Effects of biochar from different feedstock combinations and pyrolysis temperatures on basic cation contents in an acid soil | |||
| Biochar Feedstock | Ex. Ca (cmol kg–1) ------------------------------- |
Ex. Mg (cmol kg–1) ------------------------------- |
Ex. K (cmol kg–1) ------------------------------ |
||||||
Pyrolysis temperature ------------------- |
Pyrolysis temperature ------------------- |
Pyrolysis temperature ------------------- |
|||||||
350°C |
450°C |
Mean |
350°C |
450°C |
Mean |
350°C |
450°C |
Mean |
|
| SD+PM | 4.03 |
4.62 |
4.32 |
1.4 |
1.5 |
1.45 |
0.77 |
0.8 |
0.78 |
| SD-PM | 4.06 |
3.89 |
3.97 |
1.39 |
1.4 |
1.39 |
0.71 |
0.7 |
0.7 |
| TD+PM | 4.38 |
4.06 |
4.22 |
1.49 |
1.44 |
1.46 |
0.83 |
0.73 |
0.78 |
| TD-PM | 3.67 |
3.95 |
3.81 |
1.41 |
1.44 |
1.42 |
0.7 |
0.73 |
0.71 |
| Mean | 4.03 |
4.13 |
1.42 |
1.44 |
0.75 |
0.74 |
|||
| Biochar feedstock (LSD) | 0.003*** |
0.006*** |
0.006*** |
||||||
| Pyrolysis temperature (LSD) | 0.002*** |
0.004*** |
0.004*** |
||||||
| BF×Temp (LSD) | 0.005*** |
0.009*** |
|||||||
| Checks | |||||||||
| CaCO3 | 4.88 |
1.44 |
0.81 |
||||||
| AC | 1.58 |
0.63 |
0.25 |
||||||
| SD+PM@350, SD+PM@450, TD+PM@350 and TD+PM@450 are sawdust and tithonia biochar co-pyrolyzed with poultry manure at 350 and 450°C respectively while SD-PM@350, SD-PM@450, TD-PM@350 and TD-PM@450 are sole sawdust and tithonia biochar pyrolyzed at 350 and 450°C respectively, AC is absolute control that received neither biochar nor CaCO3, *** Significant at p<0.001 and ns: Not significant | |||||||||
Effects of biochar from different feedstock combinations and pyrolysis temperatures on basic caSD+PM@350, SD+PM@450, TD+PM@350 and TD+PM@450 are sawdust and tithonia biochar co-pyrolyzed with poultry manure at 350 and 450°C respectively while SD-PM@350, SD-PM@450, TD-PM@350 and TD-PM@450 are sole sawdust and tithonia biochar pyrolyzed at 350 and 450°C respectively, AC is absolute control that received neither biochar nor CaCO3, *** Significant at p<0.001 and ns: Not significanttion contents in an acid soil: The application of different biochar types and conventional lime significantly increased exchangeable basic cation concentrations in the acid soil compared to unamended soil (Table 4). Conventional lime was superior to all the biochar types in enhancing exchangeable Ca while SD+PM at 450°C and TD+PM at 350°C outperformed CaCO3 in improving exchangeable Mg and K, respectively. All the biochar tested consistently enriched the acid soil with more than two folds’ basic cations over unamended soil. Generally, biochar co-pyrolyzed with PM enriched the acid soil with higher concentrations of the basic cations compared to sole SD and TD biochar types. This variation was also significantly influenced by pyrolysis temperature such that biochar produced at a higher pyrolysis temperature of 450°C supported higher mean exchangeable Ca and Mg in the amended soils across the different biochar types. The higher basic nutrient contents in PM resulting from the nutrition the poultry birds were fed contributed significantly to higher PM capacity to mineralize higher concentrations of basic ions into the soils they were amended with. This is consistent with the submissions of Lauricella et al.30.
| Table 5: | Effects of biochar from different feedstock combinations and pyrolysis temperatures on organic carbon, exchangeable acidity and available P in an acid soil | |||
| Biochar feedstock | Organic carbon (%) ------------------------------- |
Ex. Acidity (cmol kg–1) ------------------------------- |
Available P (mg kg–1) ------------------------------ |
||||||
Temperature ------------------- |
Temperature ------------------- |
Temperature ------------------- |
|||||||
| 350°C | 450°C | Mean | 350°C | 450°C | Mean | 350°C | 450°C | Mean | |
| SD+PM | 3.67 | 3.66 | 3.66 | 1.26 | 1.2 | 1.23 | 11.66 | 15.37 | 13.52 |
| SD-PM | 3.69 | 3.7 | 3.69 | 1.32 | 1.35 | 1.34 | 10.1 | 8.97 | 9.53 |
| TD+PM | 3.64 | 3.68 | 3.66 | 1.21 | 1.23 | 1.22 | 13.53 | 12.29 | 12.91 |
| TD-PM | 3.7 | 3.68 | 3.69 | 1.45 | 1.48 | 1.47 | 9.4 | 12.13 | 10.77 |
| Mean | 3.67 | 3.68 | 1.31 | 1.32 | 11.17 | 12.19 | |||
| Biochar feedstock (LSD) | 0.003*** | 0.005*** | 0.027*** | ||||||
| Temperature (LSD) | 0.002*** | 0.004** | 0.019*** | ||||||
| BFS×Temp (LSD) | 0.005*** | 0.008*** | 0.038*** | ||||||
| Checks | |||||||||
| CaCO3 | 1.44 | 1.42 | 9.11 | ||||||
| AC | 1.46 | 2.42 | 5.88 | ||||||
| SD+PM@350, SD+PM@450, TD+PM@350 and TD+PM@450 are sawdust and tithonia biochar co-pyrolyzed with poultry manure at 350 and 450°C, respectively while SD-PM@350, SD-PM@450, TD-PM@350 and TD-PM@450 are sole sawdust and tithonia biochar pyrolyzed at 350 and 450°C, respectively, AC is absolute control that received neither biochar nor CaCO3, **,***Significant at p<0.01 and 0.001, respectively and ns: Not significant | |||||||||
Effects of biochar from different feedstock combinations and pyrolysis temperatures on organic carbon, exchangeable acidity and available P in an acid soil: Biochar application dramatically increased organic carbon contents by more than two folds compared to CaCO3-treated soil and unamended soil (Table 5). Organic C was slightly lower (1.44%) in soil amended with CaCO3 compared to unamended soil (1.46%). The sole use of CaCO3 for the management of acid soils adversely affected soil organic matter and its sole use has been indicated to have organic carbon-sapping effects in soils15,18. Biochar produced from feedstock and pyrolysis temperature which supported higher soil pH and macronutrient enrichment encouraged slightly lower SOC build-up compared to other biochar types. This indicated the inability of the same biochar types to at this stage ensure and sustain higher liming, nutrient enrichment and carbon sequestration abilities in this organic carbon and macronutrient-depleted acid soil.
Exchangeable acidity concentrations in the acid soil after cowpea seedling harvesting were affected significantly by biochar and CaCO3 applications (Table 5). Biochar treatments that supported higher exchangeable bases resulted in lower exchangeable acidity in the soil studied. This shows an appropriate exchange of the acidic cations on the soil exchangeable sites and soil solutions with the basic cations mineralized into the soil solution from the biochar. Exchangeable acidity reduced from the initial 2.20 cmol kg–1 to a range of 1.20 (SD+PM at 450°C) to 1.48 cmol kg–1 (TD-PM at 450°C) compared to 1.42 and 2.42 cmol kg–1 obtained from CaCO3 treated and unamended soils, respectively.
Acidity neutralization in acid soils following biochar application had been reported for biochar with the capacity to elevate soil pH, reduce toxic Al3+ solubility and enhance basic cation concentrations for the displacement of acidic cations on the soil exchange sites20,34. Furthermore, improvement in organic carbon status in acid soils amended with biochar has been pinpointed as an outstanding mechanism for acidity neutralization through the formation of organo mineral complex with the displaced toxic acidic cations via active functions such as the phenolic and oxalate compounds35.
The availability of phosphorus in the soil labile pool was significantly improved following the application of biochar compared to conventional and unamended soils (Table 5). Soil available P increased from the initial 3.86 mg kg–1 to a range of 8.97 (SD-PM at 450°C) to 15.37 mg kg–1 (SD+PM at 450°C). The improved soil pH from 4.35 to a range of 5.56 (TD-PM at 350°C) to 5.95 (SD+PM at 450°C) at harvesting brought about reduced Al solubility (as indicated by reduced exchangeable acidity from 2.20 cmol kg–1 to a range of 1.20 (SD+PM at 450°C) to 1.48 cmol kg–1 (TD-PM at 450°C) across biochar-amended soils) and sorption to P. The improved P in the soil was contributed from mineralization of organic P in the biochar and/or desorption from earlier fixed P by the toxic Al in the acid soil. Improved P pool following pH increases and reduced Al toxicity in biochar-amended soil through physical trapping and complex formation of the toxic acidic cations (Al3+ and H+) with oxygenated functional groups on the biochar had been documented for tropical acid soils30,36.
Effects of biochar from different feedstock combinations and pyrolysis temperatures on root parameters of cowpea seedlings in an acid soil: Root length and dry root weight of cowpea seedlings were significantly enhanced by all the biochar tested with values consistently higher than observations from CaCO3 amended soil and absolute control soil (Fig. 2 and 3). Shortening of root in terms of reduced root length and weights is a major physical defect in plants stressed by Al toxicity in acid soil12,18. Cowpea seedling root length ranged from 3.43 (TD+PM@450) to 8.87 cm (TD-PM@450) compared to 2.10 and 1.77 cm from CaCO3 and AC soils, respectively. Contrarily, cowpea dry root weight ranged from 0.04 (in SD-PM@450) to 0.15 g/plant (SD+PM@450) compared to 0.03 and 0.02 g/plant from CaCO3 and AC soils, respectively.
The cowpea seedling root parameters measured from biochar-amended soils were however not significantly different from one another. These improved cowpea seedling root parameter’s performances in biochar-amended soil are an indication of improved soil pH, macronutrient conditions and reduced Al3+ toxicity in the soil. This is consistent with the submissions of Agegnehu et al.1 and Abdulaha-Al Baquy et al.37. The multiple roles played by the biochar such as improved pH, macronutrient concentrations and organic carbon encouraged improved nutrient uptake and utilization by the test plant resulting in better cowpea root performance compared to the cowpea seedlings from soils amended with CaCO3. The CaCO3 reaction pathway was limited to only increasing soil pH after the dissolution of Ca2+ in soil moisture. The CaCO3 does not have the potential to supply the soil with any of the essential macronutrients such as N, P and K and organic carbon as observed with the different biochar tested. This is a major limitation to the use of conventional liming materials in achieving holistic acidity amelioration in tropical soils. Carbon depletion and the need for the application of additional macronutrient sources had been reported for acid soils amended with CaCO318,34,38. Its use in nutrient-degraded acid soil studied must therefore be accompanied by an additional nutrient source that will supply the essential nutrient elements after its pH neutralization if crop growth and yield would be optimized.
Correlation coefficient relating selected soil and plant parameters in an acidic soil amended with different biochar types: Soil organic carbon maintained a negative significant relationship with all the soil parameters (except exchangeable acidity) considered while its negative correlation with plant parameters was not significant (Table 6). The former correlation indicated that the SOC sequestered in the the soil over the incubation period following biochar application was utilized for macronutrient enrichment in the soils. This is supported by increases in exchangeable bases and available P concentrations in soils amended by these biochar types. These nutrient enrichments redistributed cations in the acid soil which resulted in increased soil pH. The increased nutrient enrichment was accompanied by proportionate decreases in the SOC pool, thus explaining the observed negative relationship.
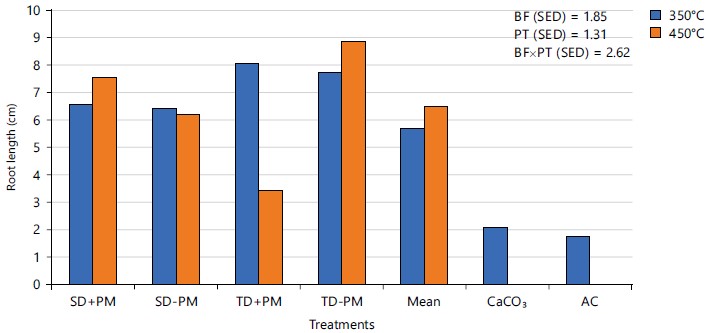
|
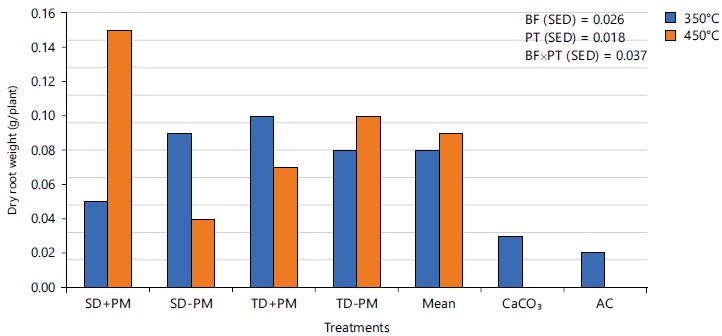
|
The increasing exchangeable bases especially Ex. Ca and K and available P enrichment were the major pathways for exchangeable acidity reduction in the soil order than physical trapping of the toxic Al3+ and H+ unto the organic matter from the biochar. This is indicated by the negative significant correlation of exchangeable acidity with Ex. Ca (r= -0.78*) and Ex. K (r= -0.70*) while its correlation with Ex. The Mg (r= -0.50) and available P (r= -0.62) was however not significant. The very low initial organic carbon (1.32%) and macronutrient status of the acid soil studied mediated this reaction pathway such that a large proportion of the organic carbon built up following biochar application was channeled first into enhancing the soil macronutrient pool. A similar observation has been reported by Zhang et al.39.
| Table 6: | Correlation coefficient relating selected soil and plant parameters in an acidic soil amended with different biochar types | |||
| Avail. P | Avail. P | Ex. Ca | DRW | Ex. Acidity | Ex. K | Ex. Mg | RL | SOC | pH |
| 1 | |||||||||
| Ex. Ca | 0.89** | 1 | |||||||
| DRW | 0.76* | 0.71* | 1 | ||||||
| Ex. Acidity | -0.62ns | -0.78* | -0.22ns | 1 | |||||
| Ex. K | 0.85** | 0.84** | 0.52ns | -0.70* | 1 | ||||
| Ex. Mg | 0.90** | 0.81** | 0.77* | -0.50ns | 0.80** | 1 | |||
| RL | 0.41ns | 0.14 | 0.58ns | 0.38ns | 0.34ns | 0.54ns | 1 | ||
| SOC | -0.85** | -0.82** | -0.51ns | 0.68* | -0.98** | -0.80** | -0.37ns | 1 | |
| pH | 0.67* | 0.67* | 0.33ns | -0.77* | 0.78* | 0.63ns | 0.09ns | -0.84** | 1 |
| *,**Significant at p<0.05 and 0.01, respectively, ns: Not significant (n = 7). Avail. P: Available phosphorus, Ex. Ca: Exchangeable Calcium, DRW: Dry root weight and RL: Root length | |||||||||
The organic carbon and exchangeable acidity statuses of the soil after cowpea seedling harvesting are showing the need for further carbon build-up in the soil to achieve a situation where further increases in SOC content will account for a negative correlation with exchangeable acidity. Higher biochar application rates (above the 5t/ha tested in this trial) will mineralize more basic cations, P and at the same time ensure higher recalcitrant carbon that will scavenge, physically trap the displaced acidic ions from the soil exchangeable sites and chelate them permanently is required for sustainable acidity amelioration of this soil. This higher biochar application rate and aging in the soil will also benefit improved cowpea seedling performance.
The non-significance of Ex. Mg correlation with exchangeable acidity following biochar application compared to Ex. Ca and K is indicating a lower contribution of Ex. Mg to the acidity neutralization in the soil. This is directly related to a lower concentration proportion of Mg in each of the biochar tested compared to higher Ca and K contents in the tested biochar types. The dominant nutrients in biochar are therefore important in determining what concentrations of these nutrients would be mineralized into the soil to achieve the targeted amelioration state.
CONCLUSION
The liming efficiencies, macronutrient enrichment and carbon sequestration potentials of biochar derived from sawdust and Tithonia diversifolia weed with or without poultry manure at 350 and 450°C pyrolysis temperatures were compared with conventional CaCO3 under incubation conditions in acid soil. Biochar derived from T. diversifolia had higher pH, ash, basic cations, N and P contents while organic carbon was higher in sawdust-derived biochars. The inclusion of poultry manure in the basal feedstock increased the liming efficiency and macronutrient (available P and basic cations) enrichment potentials of all the biochar with a greater performance at 450°C pyrolysis temperature. All the biochars had comparatively high liming efficiencies as the conventional CaCO3 but outperformed CaCO3 in their macronutrient enrichment, carbon sequestration and cowpea seedling root parameters enhancement. Increasing exchangeable Ca and K and available P enrichments was the major pathway for exchangeable acidity reduction in the acid soil studied. Widely distributed invasive weed (Tithonia diversifolia) in the study area can therefore be exploited as feedstock for biochar production to reduce over-reliance on feedstock from trees. Doing this will help achieve reduced forest tree depletion and provide an environment-friendly option for the management of invasive weeds. Finally, the need for a higher biochar application rate that would ascertain higher macronutrient enrichment with correspondingly higher organic carbon sequestration in the soil is recommended for holistic and sustainable acidity management in the soil studied.
SIGNIFICANCE STATEMENT
The study was embarked upon to provide local solutions for the management of soil acidity especially in the study areas where conventional liming materials are not readily available. Indigenous biochar was made from widely available invasive weed (Tithonia diversifolia) in the study area to discourage the continued use of woody feedstock for biochar production. This indigenous biochar outperformed conventional lime in enriching the acid soil with organic carbon and macronutrient and improving crop performance. The potential of the biochar to encourage increased concentrations of Ca, K and P was the dominant mechanism utilized for acidity amelioration in the soil studied.
REFERENCES
- Agegnehu, G., T. Amede, T. Erkossa, C. Yirga and C. Henry et al., 2021. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agric. Scand. Sect. B-Soil Plant Sci., 71: 852-869.
- Bojórquez-Quintal, E., C. Escalante-Magaña, I. Echevarría-Machado and M. Martínez-Estévez, 2017. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci., 8:01767.
- von Uexküll, H.R. and E. Mutert, 1995. Global extent, development and economic impact of acid soils. Plant Soil, 171: 1-15.
- Sanchez, P.A. and T.J. Logan, 1992. Myths and Science about the Chemistry and Fertility of Soils in the Tropics. In: Myths and Science of Soils of the Tropics, Volume 29, Lal, R. and P.A. Sanchez (Eds.), Wiley, United States, ISBN: 9780891189244, pp: 35-46.
- Iren, O.B., D.J. Udoh, V.F. Ediene and E.E. Aki, 2021. Assessment of soil properties and the development of lime requirement equations for some soils in South-Eastern Nigeria. Int. J. Soil Sci., 16: 1-12.
- Bello, O. S. and A.E. Udofia, 2013. Effects of liming on some properties of South Eastern soils of Nigeria. Niger. J. Soil Sci., 23: 124-129.
- Smyth, A.J. and R.F. Montgomery, 1962. Soils and Land use in Central Western Nigeria. Government of Western Nigeria, Ibadan, Nigeria, Pages: 265.
- Bello, A., D.B. Adie, U.A. Abubakar, A. Giwa, O.A. Adetunji and Y. Adamu, 2020. Comparative investigation on the impact of chemical and biofertilizers on some soil properties. Niger. J. Eng., 27: 36-44.
- Bunmi, O.Y. and O.J. Ajayi, 2020. The reactions of common chemical fertilizers in an ultisol and their effects on cowpea performance. J. Trop. Soils, 24: 25-32.
- Ande, O.T., J. Huising, A.O. Ojo, J. Azeez and K.S. Are et al., 2017. Status of integrated soil fertility management (ISFM) in Southwestern Nigeria. Int. J. Sustainable Agric. Res., 4: 28-44.
- Fageria, N.K. and A.S. Nascente, 2014. Management of Soil Acidity of South American Soils for Sustainable Crop Production. In: Advances in Agronomy, Sparks, D.L. (Ed.), Academic Press, United State, ISBN: 978-0-12-802139-2, pp: 221-275.
- Sade, H., B. Meriga, V. Surapu, J. Gadi, M.S.L. Sunita, P. Suravajhala and P.B.K. Kishor, 2016. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals, 29: 187-210.
- Kopittke, P.M. and F.P.C. Blamey, 2016. Theoretical and experimental assessment of nutrient solution composition in short-term studies of aluminium rhizotoxicity. Plant Soil, 406: 311-326.
- Samad, R., P. Rashid and J.L. Karmoker, 2021. Anatomical changes in chickpea (Cicer arietinum L.) under aluminium stress condition. Dhaka Univ. J. Biol. Sci., 30: 187-196.
- Mockeviciene, I., D. Karcauskiene, A. Slepetiene, M. Vilkiene, R. Repsiene, Z. Braziene and O. Anne, 2022. Influence of liming intensity on fractions of humified organic carbon in acid soil: A case study. Sustainability, 14: 5297.
- Opala, P.A., M. Odendo and F.N. Muyekho, 2018. Effects of lime and fertilizer on soil properties and maize yields in acid soils of Western Kenya. Afr. J. Agric. Res., 13: 657-663.
- Iren, O.B. and I.D. Uwah, 2018. Effects of local liming materials on soil properties and yield of waterleaf (Talinum fructicosum (L.) Juss.) in an ultisol of Southeast Nigeria. World News Nat. Sci., 21: 53-63.
- An-Ning, G., D. Gui-Lan, Z. Zhong-Qiu, T. Zhong, W. Yang-Yang and Wang Bo-Xun, 2017. Effects of CaCO3 application on soil microbial nitrogen cycle in an acid soil. Chin. J. Environ. Sci., 8: 3483-3488.
- Oyeyiola, Y.B. and J.A.I. Omueti, 2016. Phosphorus uptake and use efficiency by cowpea in phosphocompost and chemical fertilizer treated nutrient degraded acid soils. Agric. Res. Technol. Open Access J., 1.
- Agegnehu, G. and T. Amede, 2017. Integrated soil fertility and plant nutrient management in tropical agro-ecosystems: A review. Pedosphere, 27: 662-680.
- Amede, T. and A.M. Whitbread, 2022. Restoring degraded landscapes and fragile food systems in Sub-Saharan Africa: Synthesis of best practices. Renewable Agric. Food Syst., 37: S1-S3.
- Omotade, İ., S. Momoh, B. Oluwafemi and E. Agboola, 2020. Comparative analysis of nutrients composition in biochar produced from different feedstocks at varying pyrolysis temperature. Environ. Res. Technol., 3: 64-70.
- Soil Science Division Staff, 2017. Soil Survey Manual. 18th Edn., United States Department of Agriculture, Washington, D.C., Pages: 603.
- Amusan, A.A. and T.I. Ashaye, 1991. Granitic-gneiss derived soils in humid forest-tropical Southwesten Nigeria I: Genesis and classification. Ife J. Agric., 13: 1-10.
- Walkley, A. and I.A. Black, 1934. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci., 37: 29-38.
- Juo, A.S.R., 1978. Selected Methods for Soil and Plant Analysis. 2nd Edn., International Institute of Tropical Agriculture, Ibadan, Nigeria, Pages: 52.
- Oyeyiola, Y.B., E.A. Ewetola, O.E. Olaitan and M.I. Arogundade, 2021. Biochar yield and quality enhancement by poultry manure spiking at varied slow pyrolysis heating regimes. J. Solid Waste Technol. Manage., 47: 705-716.
- Anetor, M.O. and E.A. Akinrinde, 2007. Lime effectiveness of some fertilizers in a tropical acid alfisol. J. Cent. Eur. Agric., 8: 17-24
- Zhelezova, A., H. Cederlund and J. Stenström, 2017. Effect of biochar amendment and ageing on adsorption and degradation of two herbicides. Water Air Soil Pollut., 228: 216.
- Lauricella, D., Z. Weng, G.J. Clark, C.R. Butterly and G. Li et al., 2021. Biochars and their feedstocks differ in their short-term effects in ameliorating acid soils grown with aluminium-sensitive wheat. J. Soils Sediments, 21: 2805-2816.
- Mehmood, K., J.Y. Li, J. Jiang, R.Y. Shi, Z.D. Liu and R.K. Xu, 2017. Amelioration of an acidic ultisol by straw-derived biochars combined with dicyandiamide under application of urea. Environ. Sci. Pollut. Res., 24: 6698-6709.
- Geng, N., X. Kang, X. Yan, N. Yin and H. Wang et al., 2022. Biochar mitigation of soil acidification and carbon sequestration is influenced by materials and temperature. Ecotoxicol. Environ. Saf., 232: 113241.
- Wan, Q., J.H. Yuan, R.K. Xu and X.H. Li, 2014. Pyrolysis temperature influences ameliorating effects of biochars on acidic soil. Environ. Sci. Pollut. Res., 21: 2486-2495.
- Mensah, A.K. and K.A. Frimpong, 2018. Biochar and/or compost applications improve soil properties, growth, and yield of maize grown in acidic rainforest and coastal savannah soils in Ghana. Int. J. Agron., 2018: 6837404.
- Wang, H., R.F. Chen, T. Iwashita, R.F. Shen and J.F. Ma, 2015. Physiological characterization of aluminum tolerance and accumulation in tartary and wild buckwheat. New Phytol., 205: 273-279.
- Dong, Y., H. Wang, E. Chang, Z. Zhao, R. Wang, R. Xu and J. Jiang, 2019. Alleviation of aluminum phytotoxicity by canola straw biochars varied with their cultivating soils through an investigation of wheat seedling root elongation. Chemosphere, 218: 907-914.
- Abdulaha-Al Baquy, M., J.Y. Li, C.Y. Xu, K. Mehmood and R.K. Xu, 2017. Determination of critical pH and Al concentration of acidic Ultisols for wheat and canola crops. Solid Earth, 8: 149-159.
- Amare, B., E. Molla, Y.G. Selassie, H.T. Belay and T. Bayu, 2022. Effects of different dose of nitrogen and lime on soil properties and maize (Zea mays L.) on acidic nitisols of Northwestern Ethiopia. J. Agric. Nat. Res., 5: 213-227.
- Zhang, R., Z. Qu, L. Liu, W. Yang, L. Wang, J. Li and D. Zhang, 2022. Soil respiration and organic carbon response to biochar and their influencing factors. Atmosphere, 13: 2038.
How to Cite this paper?
APA-7 Style
Oyeyiola,
Y.B., Ogunlaran,
L.A. (2023). Soil Acidity Ameliorative Potentials of Biochar from Sawdust and Tithonia diversifolia Feedstock. Trends in Agricultural Sciences, 2(3), 298-309. https://doi.org/10.17311/tas.2023.298.309
ACS Style
Oyeyiola,
Y.B.; Ogunlaran,
L.A. Soil Acidity Ameliorative Potentials of Biochar from Sawdust and Tithonia diversifolia Feedstock. Trends Agric. Sci 2023, 2, 298-309. https://doi.org/10.17311/tas.2023.298.309
AMA Style
Oyeyiola
YB, Ogunlaran
LA. Soil Acidity Ameliorative Potentials of Biochar from Sawdust and Tithonia diversifolia Feedstock. Trends in Agricultural Sciences. 2023; 2(3): 298-309. https://doi.org/10.17311/tas.2023.298.309
Chicago/Turabian Style
Oyeyiola, Yetunde, Bunmi, and Lydia Abidemi Ogunlaran.
2023. "Soil Acidity Ameliorative Potentials of Biochar from Sawdust and Tithonia diversifolia Feedstock" Trends in Agricultural Sciences 2, no. 3: 298-309. https://doi.org/10.17311/tas.2023.298.309

This work is licensed under a Creative Commons Attribution 4.0 International License.




