Perinatal Thermal Conditioning of Three Broiler Strains in Southwestern Nigeria
| Received 11 Dec, 2022 |
Accepted 23 Mar, 2023 |
Published 30 Jun, 2023 |
Background and Objective: Heat stress resulting from climate change has increasingly challenged the sustainability of poultry production in the tropics. This study determined the effect of early-age thermal conditioning in selected local and exotic chickens in Nigeria. Materials and Methods: On day six, twenty chicks each from Cobb 500 (C500), Ross 308 (R308) and improved Nigerian indigenous broiler-FUNAAB Alpha (FA) strains were thermally conditioned at 40±1°C for 3 hrs. Conditioned and unexposed chicks were acutely challenged at 40±1°C for 15 min on day ten, just before collecting blood and tissue samples for haematology and qPCR, respectively. Results: Thermal conditioning significantly (p<0.05) lowered all heat stress indices in this study. Significance (p<0.05) was observed on haematological parameters and BDNF gene expression by interactive effect of strain and thermal conditioning. The highest means for packed cell volume, haemoglobin and red blood cell counts were recorded in conditioned C500 while conditioned FA had the highest expression of BDNF. Conclusion: This study showed that response to perinatal heat conditioning in chickens is strain-specific. To tackle climate change effects in the southwestern part of Nigeria and generally, in the tropics, it is recommended that farmers thermally condition commercial broiler chicks.
| Copyright © 2023 Folarin et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Global warming has aggravated heat stress-related problems in poultry production1. While demand for livestock products is expected to continue to increase globally2,3, the growth and meat quality of broilers has been negatively affected by heat stress4.
Chicken thermoregulation, on which individual bird’s response to thermal stress depends5-8 has been revealed to be heavily influenced by genetic or strain differences.
Acquisition of thermo-tolerance, which enables broiler chickens to adapt to extreme heat due to climate change, has been found to be enhanced by thermal conditioning, which is achieved by exposing poultry species to high ambient temperatures during critical periods9.
It has been verified that the Brain-Derived Neurotrophic Factor activates the pathway that leads to the establishment of thermal stress response threshold in birds. Also, short-term differences are observable in the expression of the gene during both heat conditioning and re-exposure of conditioned chicks to heat stress, when compared to its expression in unconditioned chicks of the same age10-11. It has been reported that memory, adaptation and survival are improved by higher expressions of the BDNF gene12-13.
Farmers raising commercial broilers, especially in the tropical regions of the world need guidance in making profitable strain or breed selection and mitigation of the effects of heat stress due to climate change. Information on the verification of the interactive effect of strain and thermal treatment at critical periods on thermoregulation and thermo-tolerance of individual chickens was not available as of when this study was conducted, to the best of the author’s knowledge. This study, therefore, determined the effect of perinatal thermal conditioning on heat stress indices, haematological parameters and expression of Brain-Derived Neurotrophic Factor (BDNF) gene in Cobb 500 (C500), Ross 308 (R308) and improved Nigerian indigenous broiler-FUNAAB Alpha (FA).
MATERIALS AND METHODS
Experimental birds: Fifty days old chicks each of Cobb 500 and Ross 308, which were obtained from Zartech Farms and Agrited Nigeria Limited, respectively in Ibadan, as well as the improved Nigerian indigenous broiler-FUNAAB Alpha, obtained from the Federal University of Agriculture, Abeokuta (FUNAAB) hatchery, Nigeria. For the ten days of the experiment, which was conducted in November, 2016, they were provided with a conventional starter mash (23% crude protein, 2100 kcal metabolisable energy) and water ad libitum.
Experimental location: The field study was conducted in Abeokuta, Nigeria. The experimental birds were raised in partitioned brooder cages at an environmental temperature of 32±2°C and relative humidity of 70-80% under continuous artificial lighting14-15. Benchwork was carried out at Africa Biosciences, Ibadan, Nigeria.
Thermal conditioning and acute thermal challenge: On day six, chicks from each strain were randomly distributed into two treatment groups (conditioned and unconditioned) of twenty-five birds each. While the unconditioned group were left at normal brooding temperature, the conditioned group were exposed to high temperature at 40±1°C for 3 hrs. This study adopted day 6 for thermal conditioning based on reports by researchers16,17. On day ten, both groups were exposed to acute thermal challenge at 40±1°C for 15 min without feed and water17.
Data collection: Rectal temperatures and respiratory rates of each bird (before and immediately after the acute heat challenge) were measured using a digital thermometer and stopwatch, respectively.
Immediately after the acute heat challenge, about 2 mL of blood was collected from ten birds from each group for all three strains via cardiac puncture17. The blood samples were dispensed into clean bijou bottles with Ethylenediaminetetraacetic Acid (EDTA) as an anticoagulant, for determining the Packed Cell Volume (PCV), Haemoglobin concentration (Hb), Red Blood Cell (RBC) and white blood cell differential (heterophils, lymphocytes, basophils and eosinophils) counts.
| Table 1: | Primer sequences used for quantitative real-time PCR in this study | |||
| Gene | Forward primer (F) | Reverse primer (R) |
| BDNF | 5'-TGGGTAACAGCAGCGGAGAA-3' | 5'-TATTGCTTCAGTTGGCCTTTAG-3' |
| GAPDH | 5'-AAGGAGTGAGCCAAGCACACA-3' | 5'-TCACTGCAGGATGCAGAACTG-3' |
| β-Actin | 5'-CCCAAAGCCAACAGAGAGAAG-3' | 5'-ACCATCACCAGAGTCCATCAC-3' |
| BDNF: Brain-derived neurotrophic factor, GAPDH: Glycealdehyde-3-phosphate dehydrogenase and Source: Byerly et al.18 | ||
Also, from each strain, five random chicks each from the conditioned and unconditioned groups were slaughtered by cervical dislocation. From the anterior hypothalamus of each bird, 0.5 g tissue samples were collected and stored immediately in Eppendorf tubes containing 2.5 μL RNA later solution (i.e. 1:5) and stored at -40°C.
Total RNA isolation from each stored sample was carried out using Norgen’s Animal Tissue RNA purification kit while Norgen’s TruScript™ First Strand cDNA Synthesis Kit was used for the First-Strand cDNA Synthesis reaction. The Norgen products, including the RNA later solution, were supplied by Norgen Biotek Corp., Thoroid, Ontario, Canada.
The real-time polymerase chain reaction of cDNA products was performed using the Cepheid SmartCycler, manufactured by Cepheid, Sunnyvale, California, USA. Relative quantitation of the cDNA for the BDNF gene was carried out using a 5X EvaGreen master mix (manufactured by Solis Biodyne) containing DNA Polymerase, dNTPs, MgCl2 and EvaGreen dye. The total reaction mixture of 20 μL contained 4 μL master mix, 0.3 μL forward and reverse primers (Table 1), 2 μL template and 13.4 μL PCR grade water.
Based on the result of an initial evaluation using gel electrophoresis, β-Actin was selected as the endogenous control gene. In compliance with the recommendation of the kit manufacturer, in order to activate the DNA polymerase in the master mix, an initial incubation step of 12 min at 95°C was carried out at the beginning of the qPCR cycle. Following this were 40 cycles of three qPCR steps of denaturation at 95°C for 15 sec, annealing (calculated for each primer from their melting temperatures) for 20 sec and extension at 72°C for 20 sec. Samples were run in duplicates and the averages were recorded to enhance the reliability of the results as shown in Table 1.
Statistical analysis: To relatively quantify BDNF gene expression, the ΔCT value for each sample was obtained by subtracting the CT value of β-actin (reference gene) from the CT of BDNF (target gene). The ΔΔCT was generated by subtracting ΔCT for the control group from ΔCT of each experimental sample19. The approximate fold difference, 2–ΔΔCT values, were calculated using the ΔΔCT.
Data obtained for heat stress and haematological parameters as well as the approximate fold difference (2–ΔΔCT) values from gene expression data were analyzed using the General Linear Model of SAS 9.0. Duncan’s Multiple Range Test was used for means separation20 and the results presented as Means±Standard Error (SE), at a 5% level of significance.
RESULTS
The effect of strain was significant on rectal temperature before acute heat exposure (RT1), rectal temperature after acute heat challenge (RT2), respiratory rate before acute heat challenge (RR1) and respiratory rate after acute heat challenge (RR2) as shown in Table 2. Thermal conditioning significantly (p<0.05) lowered all heat stress parameters in this study. Comparison among the six treatments resulting from the combination of the strain and thermal conditioning showed significant (p<0.05) differences in the four parameters.
The haematological profile of the three strains were statistically similar. Thermal conditioning significantly (p<0.05) increased heterophil/lymphocyte ratio (H/LR) (Table 3).
| Table 2: | Effects of chicken strain, thermal conditioning and the interaction of strain and thermal conditioning on heat stress parameters (Mean±SE) | |||
| Parameter | FA | C500 | R308 | |||
| RT1 (°C) | 41.200±0.093a | 41.850±0.120a | 41.900±0.078a | |||
| RT2 (°C) | 42.700±0.109b | 43.150±0.18a | 43.100±0.105a | |||
| RR1 (counts min–1) | 97.600±1.122b | 111.500±1.132a | 111.800±1.122a | |||
| RR2 (counts min–1) | 114.400±1.046b | 124.100±1.348ab | 131.000±1.463a | |||
| Parameter | Conditioned | Unconditioned | ||||
| RT1 (°C) | 41.302±0.059b | 41.500±0.164a | ||||
| RT2 (°C) | 42.442±0.070b | 42.940±0.069a | ||||
| RR1 (count min–1) | 101.938±0.717b | 104.280±0.709a | ||||
| RR2 (count min–1) | 117.129±0.669b | 126.080±0.662a | ||||
| Parameter | FA CON | FA UNC | C CON | C UNC | R CON | R UNC |
| RT1 (°C) | 41.10±0.16b | 41.20±0.16b | 41.58±0.13ab | 41.77±0.13a | 41.65±0.13ab | 41.70±0.13a |
| RT2 (°C) | 42.00±1.12b | 42.70±1.12ab | 42.81±0.18ab | 43.29±0.18a | 42.82±1.15ab | 43.31±1.15a |
| RR1 (count min–1) | 96.40±7.16c | 98.80±7.15c | 111.00±8.15a | 112.00±8.15a | 111.0±10.16a | 112.60±10.2a |
| RR2 (count min–1) | 110.0±10.15d | 118.80±10.15c | 117.0±11.15c | 131.20±9.45a | 128.2±10.2ab | 133.80±14.2a |
| a,b,cMean along the same row with different superscripts are significantly different at the 5% (p<0.05) level, RT1: Rectal temperature before acute heat exposure, RT2: Rectal temperature after acute heat challenge, RR1: Respiratory rate before acute heat challenge, RR2: Respiratory rate after acute heat challenge, C: 500, R: R308, CON: Conditioned and UNC: Unconditioned | ||||||
| Table 3: | Effect of chicken strain and thermal conditioning on haematological parameters (Mean±SE) | |||
| Parameter | FA | C500 | R308 |
| PCV | 33.333±2.43 | 31.300±2.545 | 29.700±2.545 |
| HB | 11.040±0.806 | 10.410±0.842 | 9.950±0.842 |
| RBC | 2.525±0.188 | 2.352±0.196 | 2.255±0.196 |
| HET | 37.833±1.688 | 39.600±1.763 | 36.400±1.763 |
| LYM | 58.250±1.555 | 56.700±1.624 | 59.700±1.624 |
| H/LR | 0.658±0.049 | 0.713±0.051 | 0.624±0.051 |
| EOS | 3.250±0.311 | 2.800±0.325 | 2.900±0.325 |
| MCV | 13.200±1.018 | 13.191±0.618 | 13.130±0.225 |
| MCHC | 0.332±0.006 | 0.333±0.004 | 0.336±0.004 |
| MCH | 4.356±0.283 | 4.394±0.164 | 4.407±0.047 |
| Parameter | Conditioned | Unconditioned | |
| PCV | 31.707±1.555 | 28.200±1.610 | |
| HB | 10.567±0.515 | 9.428±0.533 | |
| RBC | 2.334±0.120 | 2.140±0.124 | |
| HET | 41.060±1.077a | 36.760±1.115b | |
| LYM | 55.680±0.992b | 59.360±1.027a | |
| H/LR | 0.755±0.031a | 0.629±0.032b | |
| EOS | 2.693±0.199 | 2.800±0.20 | |
| MCV | 14.379±4.9283 | 13.111±0.697 | |
| MCHC | 0.333±0.0041 | 0.335±0.0051 | |
| MCH | 4.793±1.6408 | 4.393±0.2143 | |
| a,bMean along the same row with different superscripts are significantly different at the 5% (p<0.05) level, PCV: Packed Cell Volume (%), HB: Haemoglobin (g dL–1), RBC: Red Blood Cell (%), HET: Heterophil (%), LYM: Lymphocytes (%), H/LR: Heterophil/lymphocyte ratio, EOS: Eosinophil (%), MCV: Mean corpuscular volume, MCHC: Mean corpuscular haemoglobin and MCH: Mean corpuscular haemoglobin concentration | |||
The interactive effect of strain and thermal conditioning was significant (p<0.05) on all haematological parameters was shown in Table 4.
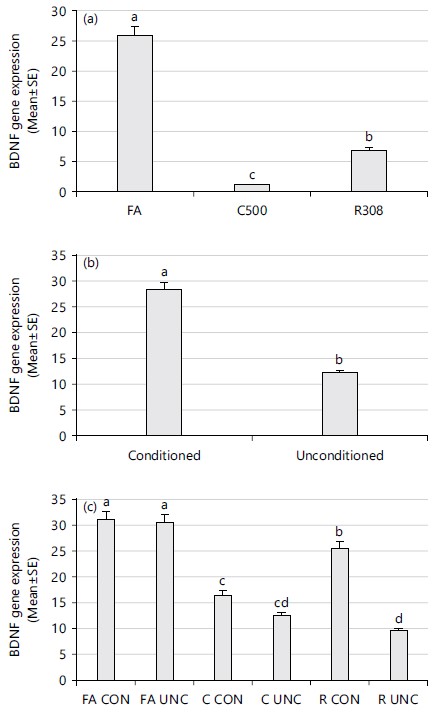
The BDNF gene was most expressed in FA and least expressed in C500 strain (Fig. 1a). Thermal conditioning significantly (p<0.05) increased BDNF expression (Fig. 1b). The interactive effect of strain and thermal conditioning was also significant (p<0.05), with unconditioned C500 having the least and conditioned FA the highest expression of BDNF gene (Fig. 1c). However, there was no statistical difference between conditioned and unconditioned FA.
|
| Table 4: | Interactive effect of strain and thermal conditioning on haematology (Mean±SE) | |||
| Parameter | FA CON |
FA UNC |
C CON |
C UNC |
R CON |
R UNC |
| PCV | 33.67±2.43ab |
33.00±2.32ab |
37.20±2.48a |
25.40±2.33b |
25.40±2.33b |
26.00±2.58b |
| HB | 11.10±0.796ab |
10.98±0.832ab |
12.34±0.716a |
8.48±0.806b |
8.48±0.806b |
8.68±0.806b |
| RBC | 2.47±0.208b |
2.58±0.223b |
2.78±0.223a |
1.92±0.223bc |
1.92±0.223bc |
1.96±0.223bc |
| HET | 37.66±2.32b |
38.00±2.41b |
43.00±2.32ab |
36.20±2.34bc |
36.20±2.34bc |
35.00±2.34c |
| LYM | 58.50±1.624ab |
58.00±1.555b |
53.40±1.555bc |
60.00±1.624a |
60.00±1.624a |
60.60±1.624a |
| HLR | 0.65±0.037ab |
0.66±0.039ab |
0.82±0.03a |
0.61±0.029b |
0.61±0.029b |
0.60±0.032b |
| EOS | 3.50±0.31a |
3.00±0.23ab |
2.40±0.23b |
3.20±0.23ab |
3.20±0.23ab |
3.00±0.23ab |
| MCV | 13.69±0.335b |
12.6±0.335b |
13.39±0.335b |
12.99±0.345b |
12.99±0.345b |
13.14±0.433b |
| MCHC | 0.333±0.03ab |
0.329±0.02b |
0.332±0.11ab |
0.34±0.03a |
0.34±0.03a |
0.34±0.03a |
| MCH | 4.50±0.04b |
4.21±0.04b |
4.44±0.133b |
4.35±0.133b |
4.35±0.133b |
4.4±0.061b |
| a,b,cMean along the same row with different superscripts are significantly different at the 5% (p<0.05) level, PCV: Packed Cell Volume (%), HB: Haemoglobin (g dL–1), RBC: Red Blood Cell (%), HET: Heterophil (%), LYM: Lymphocytes (%), H/LR: Heterophil/lymphocyte ratio, MONO: Monocytes (%), EOS: Eosinophil (%), MCV: Mean corpuscular volume, MCH: Mean corpuscular haemoglobin MCHC: Mean corpuscular haemoglobin concentration, FA: FUNAAB alpha, NF: Normal feather indigenous, C: C500, R: R308, CON: Conditioned and UNC: Unconditioned | ||||||
DISCUSSION
As revealed by heat stress parameters, FA was less stressed than the two exotic strains. This is likely due to genetic differences in heat tolerance of the chicken strains, FA being a local strain. In the tropics, under the same environmental conditions, indigenous chickens have been reported to adapt and resist diseases better than the exotics21,22. The reduction in heat stress parameters observed in the conditioned chicks could have been due to acclimation while the sharp increase in heat stress experienced by unconditioned chicks was due to the acute heat challenge, for which conditioned chicks were already prepared. The interactive effect further confirms that thermal conditioning alleviated the effect of acute heat exposure, especially for the exotic strains (C500 and R308). According to Kennedy et al.23, perinatal exposure of poultry to high environmental temperatures improves their thermo-tolerance.
Though the blood profiles of the three strains used in this study were revealed to be statistically similar, only the FA strain mean values for important erythrocytic and leukocytic indices fall within reference ranges24. PCV, Hb, RBC and EOS levels being higher in FA strain could indicate better health status since the transport of oxygen and absorbed nutrients involve PCV and Hb25,26. Thermal conditioning increased PCV, Hb and RBC in the two commercial strains (C500 and R308), whereas it had no effect on FA. This study has also shown that the effect of strain by thermal conditioning interaction (i.e., genotype×environment) on haematological parameters is strain-specific. This result agreed with Siegel27, who reported that various lines exhibited different responses in the same environmental conditions because of the genotype×environment interaction. Meanwhile, haematological parameters have been suggested for use in the selection of animals that are genetically resistant to certain environmental conditions25,28,29.
The BDNF gene is essential for synaptic plasticity and maintenance of long-term memory30, its expression has been found to be higher in thermally conditioned chicks in comparison with the unconditioned11. The difference in the expression of the gene in the three strains further affirms strain differences in chicken thermoregulation, which affects how individual birds respond to thermal stress7,8. The higher expression of the BDNF gene in conditioned birds agrees with the findings of Labunskay and Meiri10 and Yossifoff et al.11 and confers greater developmental plasticity which could make the thermally conditioned birds more thermo-tolerant when exposed to heat stress at marketing age12,13. However, the interactive effect of strain and thermal treatment suggests that the effect of perinatal thermal treatment is strain-specific since the improved indigenous strain had the highest expression of the BDNF gene and showed no statistical difference in the conditioned and unconditioned treatments. This possibly also explains why it has lower values for heat stress parameters which are indicators of thermal stress.
Based on this study, thermal conditioning of chickens is recommended for the alleviation of heat stress, especially in commercial broilers to be raised in Nigeria. This will help farmers to avoid losses and maximize profit in the face of increasing climate change effects. The molecular aspect of this study faced the challenges of limited funding, equipment and reagents. The number of chicken strains assessed was also limited. Hence, it is recommended that global gene expression profiling of thermoregulatory genes be carried out for a more robust assessment of thermal conditioning in tropical chicken strains.
CONCLUSION
Early-age thermal conditioning is beneficial for alleviating thermal stress in broiler or meat-type chickens raised in hot climates. In this study, the improved indigenous strain had an edge over exotic commercial strains in terms of heat tolerance. However, thermal conditioning enhanced thermo-tolerance in all the strains.
SIGNIFICANCE STATEMENT
This study was conducted to determine the effect of perinatal thermal conditioning on heat stress indices, haematological parameters and expression of the Brain-Derived Neurotrophic Factor (BDNF) gene in commercial broiler chicken strains in Nigeria. To the authors’ best knowledge, this information was unavailable at the time of conducting the research. The study showed that perinatal thermal conditioning reduced the stress indices measured and is therefore recommended to help farmers mitigate the effects of climate change in tropical regions.
ACKNOWLEDGMENTS
The authors wish to acknowledge and appreciate the efforts of Dr. Paul Akinduti of Covenant University and Mr. Timilehin Alakoya in the areas of technical guidance as well as laboratory and statistical analyses. They are also grateful to the National Centre for Genetic Resources and Biotechnology, particularly the Molecular/Biotechnology Laboratory, for hosting Folarin, I.A. for an internship.
REFERENCES
- Renaudeau, D., A. Collin, S. Yahav, V. de Basilio, J.L. Gourdine and R.J. Collier, 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal, 6: 707-728.
- Thornton, P.K., 2010. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B: Biol. Sci., 365: 2853-2867.
- Rojas-Downing, M.M., A.P. Nejadhashemi, T. Harrigan and S.A. Woznicki, 2017. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manage., 16: 145-163.
- Lara, L.J. and M.H. Rostagno, 2013. Impact of heat stress on poultry production. Animal, 3: 356-369.
- Rajkumar, U., B.L.N. Reddy, K.S. Rajaravindra, M. Niranjan and T.K. Bhattacharya et al., 2010. Effect of naked neck gene on immune competence, serum biochemical and carcass traits in chickens under a tropical climate. Asian-Australas. J. Anim. Sci., 23: 867-872.
- Wang, Y., P. Saelao, K. Chanthavixay, R. Gallardo and D. Bunn et al., 2018. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci., 97: 770-780.
- Altan, Ö., A. Pabuçcuoğlu, A. Altan, S. Konyalioğlu and H. Bayraktar, 2003. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci., 44: 545-550.
- Nielsen, B.L., M.G. Thomsen, P. S⊘rensen and J.F. Young, 2003. Feed and strain effects on the use of outdoor areas by broilers. Br. Poult. Sci., 44: 161-169.
- Yahav, S. and J.P. McMurtry, 2001. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life-the effect of timing and ambient temperature. Poult. Sci., 80: 1662-1666.
- Labunskay, G. and N. Meiri, 2006. R-Ras3/(M-Ras) is involved in thermal adaptation in the critical period of thermal control establishment. J. Neurobiol., 66: 56-70.
- Yossifoff, M., T. Kisliouk and N. Meiri, 2008. Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. Eur. J. Neurosci., 28: 2267-2277.
- Johnston, A.N.B., M.P. Clements and S.P.R. Rose, 1999. Role of brain-derived neurotrophic factor and presynaptic proteins in passive avoidance learning in day-old domestic chicks. Neuroscience, 88: 1033-1042.
- Johnston, A.N.B. and S.P.R. Rose, 2001. Memory consolidation in day-old chicks requires BDNF but not NGF or NT-3; an antisense study. Mol. Brain Res., 88: 26-36.
- Gan, J.K., L.Y. Jiang, L.N. Kong, X.Q. Zhang and Q.B. Luo, 2015. Analysis of genetic diversity of the heat shock protein 70 gene on the basis of abundant sequence polymorphisms in chicken breeds. Genet. Mol. Res., 14: 1538-1545.
- Yahav, S., 2009. Alleviating heat stress in domestic fowl: Different strategies. World's Poult. Sci. J., 65: 719-732.
- de Basilio, V., F. Requena, A. Leon, M. Vilarino and M. Picard, 2003. Early age thermal conditioning immediately reduces body temperature of broiler chicks in a tropical environment. Poult. Sci., 82: 1235-1241.
- Tanizawa, H., J.I. Shiraishi, S.I. Kawakami, M. Tsudzuki and T. Bungo, 2014. Effect of short-term thermal conditioning on physiological and behavioral responses to subsequent acute heat exposure in chicks. J. Poult. Sci., 51: 80-86.
- Byerly, M.S., J. Simon, E. Lebihan-Duval, M.J. Duclos, L.A. Cogburn and T.E. Porter, 2009. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol., 296: R1180-R1189.
- Livak, K.J. and T.D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408.
- Gomez, K.A. and A.A. Gomez, 1984. Statistical Procedures for Agricultural Research. 2nd Edn., John Wiley and Sons, USA, ISBN-13: 9780471870920, Pages: 704.
- Ajayi, F.O., 2010. Nigerian indigenous chicken: A valuable genetic resource for meat and egg production. Asian J. Poult. Sci., 4: 164-172
- Padhi, M.K., 2016. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica, 2016: 2604685.
- Kennedy, G.M., J.K. Lichoti and S.C. Ommeh, 2022. Heat stress and poultry: Adaptation to climate change, challenges and opportunities for genetic breeding in Kenya. J. Agric. Sci. Technol., 21: 49-61.
- Onyishi, G.C., C.C. Oguine, S.I. Nwani, I.O. Aguzie and C.D. Nwani, 2017. Haematological parameters dynamics of developing Gallus gallus domesticus. Anim. Res. Int., 14: 2769-2776.
- Etim, N.N., G.E. Enyenihi, U. Akpabio and E.E.A. Offiong, 2014. Effects of nutrition on haematology of rabbits: A review. Eur. Sci. J., 10: 413-424.
- Olumide, M.D., G.O. Chioma, O.A. Ajayi and O.E. Akinboye, 2018. Performance, haematological and serum biochemical profile of broilers chicken fed diets supplemented with Ocimum gratissimum meal. Int. J. Mod. Biol. Res., 6: 27-34.
- Siegel, H.S., 1980. Physiological stress in birds. BioScience, 30: 529-534.
- Ovuru, S.S. and I.K.E. Ekweozor, 2004. Haematological changes associated with crude oil ingestion in experimental rabbits. Afr. J. Biotechnol., 3: 346-348.
- Mmereole, F.U.C., 2008. The effects of replacing groundnut cake with rubber seed meal on the haematological and serological indices of broilers. Int. J. Poult. Sci., 7: 622-624.
- Barco, A., S. Patterson, J.M. Alarcon, P. Gromova, M. Mata-Roig, A. Morozov and E.R. Kandel, 2005. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron, 48: 123-137.
How to Cite this paper?
APA-7 Style
Folarin,
I.A., Olowofeso,
O., Ikeobi,
C.O., Akinyemi,
O.D., Oduoye,
O.T., Ilori,
B.M., Wheto,
M. (2023). Perinatal Thermal Conditioning of Three Broiler Strains in Southwestern Nigeria. Trends in Agricultural Sciences, 2(2), 161-168. https://doi.org/10.17311/tas.2023.161.168
ACS Style
Folarin,
I.A.; Olowofeso,
O.; Ikeobi,
C.O.; Akinyemi,
O.D.; Oduoye,
O.T.; Ilori,
B.M.; Wheto,
M. Perinatal Thermal Conditioning of Three Broiler Strains in Southwestern Nigeria. Trends Agric. Sci 2023, 2, 161-168. https://doi.org/10.17311/tas.2023.161.168
AMA Style
Folarin
IA, Olowofeso
O, Ikeobi
CO, Akinyemi
OD, Oduoye
OT, Ilori
BM, Wheto
M. Perinatal Thermal Conditioning of Three Broiler Strains in Southwestern Nigeria. Trends in Agricultural Sciences. 2023; 2(2): 161-168. https://doi.org/10.17311/tas.2023.161.168
Chicago/Turabian Style
Folarin, Itunuola, Anne, Olajide Olowofeso, Christian Obiora Ndubuisi Ikeobi, Olukayode Dewunmi Akinyemi, Olusola Thomas Oduoye, Babatunde Moses Ilori, and Mathew Wheto.
2023. "Perinatal Thermal Conditioning of Three Broiler Strains in Southwestern Nigeria" Trends in Agricultural Sciences 2, no. 2: 161-168. https://doi.org/10.17311/tas.2023.161.168

This work is licensed under a Creative Commons Attribution 4.0 International License.



